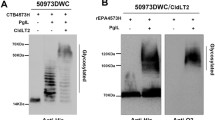
Abstract
State-of-the-art production technologies for conjugate vaccines are complex, multi-step processes. An alternative approach to produce glycoconjugates is based on the bacterial N-linked protein glycosylation system first described in Campylobacter jejuni. The C. jejuni N-glycosylation system has been successfully transferred into Escherichia coli, enabling in vivo production of customized recombinant glycoproteins. However, some antigenic bacterial cell surface polysaccharides, like the Vi antigen of Salmonella enterica serovar Typhi, have not been reported to be accessible to the bacterial oligosaccharyltransferase PglB, hence hamper development of novel conjugate vaccines against typhoid fever. In this report, Vi-like polysaccharide structures that can be transferred by PglB were evaluated as typhoid vaccine components. A polysaccharide fulfilling these requirements was found in Escherichia coli serovar O121. Inactivation of the E. coli O121 O antigen cluster encoded gene wbqG resulted in expression of O polysaccharides reactive with antibodies raised against the Vi antigen. The structure of the recombinantly expressed mutant O polysaccharide was elucidated using a novel HPLC and mass spectrometry based method for purified undecaprenyl pyrophosphate (Und-PP) linked glycans, and the presence of epitopes also found in the Vi antigen was confirmed. The mutant O antigen structure was transferred to acceptor proteins using the bacterial N-glycosylation system, and immunogenicity of the resulting conjugates was evaluated in mice. The conjugate-induced antibodies reacted in an enzyme-linked immunosorbent assay with E. coli O121 LPS. One animal developed a significant rise in serum immunoglobulin anti-Vi titer upon immunization.





Similar content being viewed by others
References
Crump, J.A., Luby, S.P., Mintz, E.D.: The global burden of typhoid fever. Bull. World Health Organ 82(5), 346–353 (2004)
Bhan, M.K., Bahl, R., Bhatnagar, S.: Typhoid and paratyphoid fever. Lancet 366(9487), 749–762 (2005)
Mirza, S.H., Beeching, N.J., Hart, C.A.: Multi-drug resistant typhoid: a global problem. J. Med. Microbiol. 44(5), 317–319 (1996)
Hone, D.M., Attridge, S.R., Forrest, B., Morona, R., Daniels, D., LaBrooy, J.T., Bartholomeusz, R.C., Shearman, D.J., Hackett, J.: A galE via (Vi antigen-negative) mutant of Salmonella typhi Ty2 retains virulence in humans. Infect. Immun. 56(5), 1326–1333 (1988)
Levine, M.M., Ferreccio, C., Black, R.E., Germanier, R.: Large-scale field trial of Ty21a live oral typhoid vaccine in enteric-coated capsule formulation. Lancet 1(8541), 1049–1052 (1987)
Black, R.E., Levine, M.M., Ferreccio, C., Clements, M.L., Lanata, C., Rooney, J., Germanier, R.: Efficacy of one or two doses of Ty21a Salmonella typhi vaccine in enteric-coated capsules in a controlled field trial. Chilean Typhoid Committee. Vaccine 8(1), 81–84 (1990)
Murphy, J.R., Grez, L., Schlesinger, L., Ferreccio, C., Baqar, S., Munoz, C., Wasserman, S.S., Losonsky, G., Olson, J.G., Levine, M.M.: Immunogenicity of Salmonella typhi Ty21a vaccine for young children. Infect. Immun. 59(11), 4291–4293 (1991)
Levine, M.M., Ferreccio, C., Cryz, S., Ortiz, E.: Comparison of enteric-coated capsules and liquid formulation of Ty21a typhoid vaccine in randomised controlled field trial. Lancet 336(8720), 891–894 (1990)
Levine, M.M., Ferreccio, C., Abrego, P., Martin, O.S., Ortiz, E., Cryz, S.: Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine 17(Suppl 2), S22–S27 (1999)
Weintraub, A.: Immunology of bacterial polysaccharide antigens. Carbohydr. Res. 338(23), 2539–2547 (2003)
Landy, M.: Studies on Vi antigen. VI. Immunization of human beings with purified Vi antigen. Am. J. Hyg. 60(1), 52–62 (1954)
Szu, S.C., Taylor, D.N., Trofa, A.C., Clements, J.D., Shiloach, J., Sadoff, J.C., Bryla, D.A., Robbins, J.B.: Laboratory and preliminary clinical characterization of Vi capsular polysaccharide-protein conjugate vaccines. Infect. Immun. 62(10), 4440–4444 (1994)
Lin, F.Y., Ho, V.A., Khiem, H.B., Trach, D.D., Bay, P.V., Thanh, T.C., Kossaczka, Z., Bryla, D.A., Shiloach, J., Robbins, J.B., Schneerson, R., Szu, S.C.: The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N. Engl. J. Med. 344(17), 1263–1269 (2001)
Young, N.M., Brisson, J.R., Kelly, J., Watson, D.C., Tessier, L., Lanthier, P.H., Jarrell, H.C., Cadotte, N., St Michael, F., Aberg, E., Szymanski, C.M.: Structure of the N-linked glycan present on multiple glycoproteins in the Gram-negative bacterium. Campylobacter jejuni. J. Biol. Chem. 277(45), 42530–42539 (2002)
Wacker, M., Linton, D., Hitchen, P.G., Nita-Lazar, M., Haslam, S.M., North, S.J., Panico, M., Morris, H.R., Dell, A., Wren, B.W., Aebi, M.: N-linked glycosylation in Campylobacter jejuni and its functional transfer into E. coli. Science (New York, N.Y) 298(5599), 1790–1793 (2002)
Feldman, M.F., Wacker, M., Hernandez, M., Hitchen, P.G., Marolda, C.L., Kowarik, M., Morris, H.R., Dell, A., Valvano, M.A., Aebi, M.: Engineering N-linked protein glycosylation with diverse O antigen lipopolysaccharide structures in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 102(8), 3016–3021 (2005)
Wacker, M., Feldman, M.F., Callewaert, N., Kowarik, M., Clarke, B.R., Pohl, N.L., Hernandez, M., Vines, E.D., Valvano, M.A., Whitfield, C., Aebi, M.: Substrate specificity of bacterial oligosaccharyltransferase suggests a common transfer mechanism for the bacterial and eukaryotic systems. Proc. Natl. Acad. Sci. U. S. A. 103(18), 7088–7093 (2006)
Kowarik, M., Young, N.M., Numao, S., Schulz, B.L., Hug, I., Callewaert, N., Mills, D.C., Watson, D.C., Hernandez, M., Kelly, J.F., Wacker, M., Aebi, M.: Definition of the bacterial N-glycosylation site consensus sequence. EMBO J. 25(9), 1957–1966 (2006)
Szu, S.C., Li, X.R., Stone, A.L., Robbins, J.B.: Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect. Immun. 59(12), 4555–4561 (1991)
Szu, S.C., Bystricky, S.: Physical, chemical, antigenic, and immunologic characterization of polygalacturonan, its derivatives, and Vi antigen from Salmonella typhi. Methods Enzymol. 363, 552–567 (2003)
Whitfield, C.: Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 75, 39–68 (2006)
Parolis, H., Parolis, L.A., Olivieri, G.: Structural studies on the Shigella-like Escherichia coli O121 O-specific polysaccharide. Carbohydr. Res. 303(3), 319–325 (1997)
King, J.D., Vinogradov, E., Tran, V., Lam, J.S.: Biosynthesis of uronamide sugars in Pseudomonas aeruginosa O6 and Escherichia coli O121 O antigens. Environ. Microbiol. 12(6), 1531–1544 (2010)
Liu, D., Reeves, P.R.: Escherichia coli K12 regains its O antigen. Microbiology 140(Pt 1), 49–57 (1994)
Feldman, M.F., Marolda, C.L., Monteiro, M.A., Perry, M.B., Parodi, A.J., Valvano, M.A.: The activity of a putative polyisoprenol-linked sugar translocase (Wzx) involved in Escherichia coli O antigen assembly is independent of the chemical structure of the O repeat. J. Biol. Chem. 274(49), 35129–35138 (1999)
Ihssen, J., Kowarik, M., Dilettoso, S., Tanner, C., Wacker, M., Thony-Meyer, L.: Production of glycoprotein vaccines in Escherichia coli. Microb. Cell. Fact. 9, 61 (2010)
Kubler-Kielb, J., Coxon, B., Schneerson, R.: Chemical structure, conjugation, and cross-reactivity of Bacillus pumilus Sh18 cell wall polysaccharide. J. Bacteriol. 186(20), 6891–6901 (2004)
Kubler-Kielb, J., Vinogradov, E., Hu, H., Leppla, S.H., Robbins, J.B., Schneerson, R.: Saccharides cross-reactive with Bacillus anthracis spore glycoprotein as an anthrax vaccine component. Proc. Natl. Acad. Sci. U. S. A. 105(25), 8709–8712 (2008)
Friedman, A.M., Long, S.R., Brown, S.E., Buikema, W.J., Ausubel, F.M.: Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene 18(3), 289–296 (1982)
Laemmli, U.K., Favre, M.: Maturation of the head of bacteriophage T4. I. DNA packaging events. J. Mol. Biol. 80(4), 575–599 (1973)
Tsai, C.M., Frasch, C.E.: A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 119(1), 115–119 (1982)
Glover, K.J., Weerapana, E., Imperiali, B.: In vitro assembly of the undecaprenylpyrophosphate-linked heptasaccharide for prokaryotic N-linked glycosylation. Proc. Natl. Acad. Sci. U. S. A. 102(40), 14255–14259 (2005)
Bigge, J.C., Patel, T.P., Bruce, J.A., Goulding, P.N., Charles, S.M., Parekh, R.B.: Nonselective and efficient fluorescent labeling of glycans using 2-amino benzamide and anthranilic acid. Anal. Biochem. 230(2), 229–238 (1995)
Merry, A.H., Neville, D.C., Royle, L., Matthews, B., Harvey, D.J., Dwek, R.A., Rudd, P.M.: Recovery of intact 2-aminobenzamide-labeled O-glycans released from glycoproteins by hydrazinolysis. Anal. Biochem. 304(1), 91–99 (2002)
Royle, L., Mattu, T.S., Hart, E., Langridge, J.I., Merry, A.H., Murphy, N., Harvey, D.J., Dwek, R.A., Rudd, P.M.: An analytical and structural database provides a strategy for sequencing O-glycans from microgram quantities of glycoproteins. Anal. Biochem. 304(1), 70–90 (2002)
Apicella, M.A.: Isolation and characterization of lipopolysaccharides. Methods Mol. Biol. 431, 3–13 (2008)
Demil, P., D’Hondt, E., Hoecke, C.V.: Salmonella Typhi vaccine compositions. European Patent EP1107787 (2003)
Hestrin, S.: The reaction of acetylcholine and other carboxylic acid derivatives with hydroxylamine, and its analytical application. J. Biol. Chem. 180(1), 249–261 (1949)
Frey, A., Di Canzio, J., Zurakowski, D.: A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 221(1–2), 35–41 (1998)
Hone, D.M., Harris, A.M., Chatfield, S., Dougan, G., Levine, M.M.: Construction of genetically defined double aro mutants of Salmonella typhi. Vaccine 9(11), 810–816 (1991)
Dykxhoorn, D.M., St Pierre, R., Linn, T.: A set of compatible tac promoter expression vectors. Gene 177(1–2), 133–136 (1996)
Acknowledgement
E. coli SCM6 was kindly provided by M. Valvano. We thank Susanne Dura und Sacha Keller for their technical assistance in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wetter, M., Kowarik, M., Steffen, M. et al. Engineering, conjugation, and immunogenicity assessment of Escherichia coli O121 O antigen for its potential use as a typhoid vaccine component. Glycoconj J 30, 511–522 (2013). https://doi.org/10.1007/s10719-012-9451-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-012-9451-9