Abstract
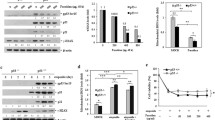
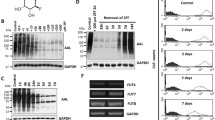
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces apoptosis in many cancer cells but not in normal ones. Recombinant TRAIL and agonistic antibodies to its cognate receptors are currently being studied as promising anticancer drugs. However, preclinical and clinical studies have shown that many types of human cancers are resistant to TRAIL agonists. We previously reported that a deficiency of fucosylation, which is one of the most common oligosaccharide modifications, leads to resistance to TRAIL-induced apoptosis. In contrast, DNA methylation is associated with silencing of various tumor suppressor genes and resistance of cancer cells to anticancer drugs. The aim of this study is to clarify the involvement of DNA methylation in the regulation of cellular fucosylation and the susceptibility to TRAIL-induced apoptosis. When nineteen cancer cell lines with relatively low fucosylation levels were treated with a novel methyltransferase inhibitor, zebularine, an increase in the fucosylation level was observed in many cancer cell lines. The expression of fucosylation-related genes, such as the FX, GDP-fucose transporter, and Fut4 genes, was significantly increased after the treatment with zebularine. Moreover, a synergistic effect of zebularine on TRAIL-induced apoptosis was observed in several cancer cell lines, in which fucosylation was increased by treatment with zebularine. This synergistic effect was independent of the expression of TRAIL receptors and caspase-8. These results indicate that cellular fucosylation is regulated through DNA methylation in many cancer cells. Moreover, zebularine might be useful as a combination drug with TRAIL-based therapies in patients with TRAIL-resistant cancer.






Similar content being viewed by others
Abbreviations
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
- AAL:
-
Aleuria aurantia lectin
- GMDS:
-
GDP-mannose-4,6-dehydratase
- AFP:
-
alpha-fetoprotein
- GDP-fucose:
-
guanosin 5′-diphosphate-fucose
- GDP-Fuc Tr:
-
GDP-fucose transporter
References
Ohtsubo, K., Marth, J.D.: Glycosylation in cellular mechanisms of health and disease. Cell 126, 855–867 (2006)
Haltiwanger, R.S., Lowe, J.B.: Role of glycosylation in development. Annu. Rev. Biochem. 73, 491–537 (2004)
Hakomori, S.: Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv. Cancer Res. 52, 257–331 (1989)
Miyoshi, E., Moriwaki, K., Nakagawa, T.: Biological function of fucosylation in cancer biology. J. Biochem. 143, 725–729 (2008)
Alpert, M.E., Uriel, J., de Nechaud, B.: Alpha-1 fetoglobulin in the diagnosis of human hepatoma. N. Engl. J. Med. 278, 984–986 (1968)
Aoyagi, Y., Isemura, M., Suzuki, Y., Sekine, C., Soga, K., Ozaki, T., Ichida, F.: Fucosylated alpha-fetoprotein as marker of early hepatocellular carcinoma. Lancet 2, 1353–1354 (1985)
Aoyagi, Y., Isemura, M., Suzuki, Y., Sekine, C., Soga, K., Ozaki, T., Ichida, F.: Change in fucosylation of alpha-fetoprotein on malignant transformation of liver cells. Lancet 1, 210 (1986)
Food and Drug Administration, HHS: Medical devices; immunology and microbiology devices; classification of AFP-L3% immunological test systems. Final rule. Fed. Regist. 70, 57748–57750 (2005)
Ohyama, C., Smith, P.L., Angata, K., Fukuda, M.N., Lowe, J.B., Fukuda, M.: Molecular cloning and expression of GDP-D-mannose-4, 6-dehydratase, a key enzyme for fucose metabolism defective in Lec13 cells. J. Biol. Chem. 273, 14582–14587 (1998)
Sullivan, F.X., Kumar, R., Kriz, R., Stahl, M., Xu, G.Y., Rouse, J., Chang, X.J., Boodhoo, A., Potvin, B., Cumming, D.A.: Molecular cloning of human GDP-mannose 4, 6-dehydratase and reconstitution of GDP-fucose biosynthesis in vitro. J. Biol. Chem. 273, 8193–8202 (1998)
Tonetti, M., Sturla, L., Bisso, A., Benatti, U., De Flora, A.: Synthesis of GDP-L-fucose by the human FX protein. J. Biol. Chem. 271, 27274–27279 (1996)
Smith, P.L., Myers, J.T., Rogers, C.E., Zhou, L., Petryniak, B., Becker, D.J., Homeister, J.W., Lowe, J.B.: Conditional control of selectin ligand expression and global fucosylation events in mice with a targeted mutation at the FX locus. J. Cell Biol. 158, 801–815 (2002)
Lübke, T., Marquardt, T., Etzioni, A., Hartmann, E., von Figura, K., Körner, C.: Complementation cloning identifies CDG-IIc, a new type of congenital disorders of glycosylation, as a GDP-fucose transporter deficiency. Nat. Genet. 28, 73–76 (2001)
Lühn, K., Wild, M.K., Eckhardt, M., Gerardy-Schahn, R., Vestweber, D.: The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat. Genet. 28, 69–72 (2001)
Noda, K., Miyoshi, E., Gu, J., Gao, C.X., Nakahara, S., Kitada, T., Honke, K., Suzuki, K., Yoshihara, H., Yoshikawa, K., Kawano, K., Tonetti, M., Kasahara, A., Hori, M., Hayashi, N., Taniguchi, N.: Relationship between elevated FX expression and increased production of GDP-L-fucose, a common donor substrate for fucosylation in human hepatocellular carcinoma and hepatoma cell lines. Cancer Res. 63, 6282–6289 (2003)
Moriwaki, K., Noda, K., Nakagawa, T., Asahi, M., Yoshihara, H., Taniguchi, N., Hayashi, N., Miyoshi, E.: A high expression of GDP-fucose transporter in hepatocellular carcinoma is a key factor for increases in fucosylation. Glycobiology 17, 1311–1320 (2007)
Okuyama, N., Ide, Y., Nakano, M., Nakagawa, T., Yamanaka, K., Moriwaki, K., Murata, K., Ohigashi, H., Yokoyama, S., Eguchi, H., Ishikawa, O., Ito, T., Kato, M., Kasahara, A., Kawano, S., Gu, J., Taniguchi, N., Miyoshi, E.: Fucosylated haptoglobin is a novel marker for pancreatic cancer: a detailed analysis of the oligosaccharide structure and a possible mechanism for fucosylation. Int. J. Cancer 118, 2803–2808 (2006)
Narisada, M., Kawamoto, S., Kuwamoto, K., Moriwaki, K., Nakagawa, T., Matsumoto, H., Asahi, M., Koyama, N., Miyoshi, E.: Identification of an inducible factor secreted by pancreatic cancer cell lines that stimulates the production of fucosylated haptoglobin in hepatoma cells. Biochem. Biophys. Res. Commun. 377, 792–796 (2008)
Wiley, S.R., Schooley, K., Smolak, P.J., Din, W.S., Huang, C.P., Nicholl, J.K., Sutherland, G.R., Smith, T.D., Rauch, C., Smith, C.A., et al.: Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3, 673–682 (1995)
Takeda, K., Hayakawa, Y., Smyth, M.J., Kayagaki, N., Yamaguchi, N., Kakuta, S., Iwakura, Y., Yagita, H., Okumura, K.: Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat. Med. 7, 94–100 (2001)
Ashkenazi, A., Herbst, R.S.: To kill a tumor cell: the potential of proapoptotic receptor agonists. J. Clin. Invest. 118, 1979–1990 (2008)
Ashkenazi, A.: Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat. Rev. Drug Discov. 7, 1001–1012 (2008)
Johnstone, R.W., Frew, A.J., Smyth, M.J.: The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat. Rev. Cancer 8, 782–798 (2008)
Moriwaki, K., Noda, K., Furukawa, Y., Ohshima, K., Uchiyama, A., Nakagawa, T., Taniguchi, N., Daigo, Y., Nakamura, Y., Hayashi, N., Miyoshi, E.: Deficiency of GMDS leads to escape from NK cell-mediated tumor surveillance through modulation of TRAIL signaling. Gastroenterology 137, 188–198 (2009)
Herman, J.G., Baylin, S.B.: Gene silencing in cancer in association with promoter hypermethylation. N Engl. J. Med. 349, 2042–2054 (2003)
Jones, P.A., Baylin, S.B.: The epigenomics of cancer. Cell 128, 683–692 (2007)
Kannagi, R., Yin, J., Miyazaki, K., Izawa, M.: Current relevance of incomplete synthesis and neo-synthesis for cancer-associated alteration of carbohydrate determinants–Hakomori’s concepts revisited. Biochim. Biophys. Acta 1780, 525–531 (2008)
Kawamura, Y.I., Toyota, M., Kawashima, R., Hagiwara, T., Suzuki, H., Imai, K., Shinomura, Y., Tokino, T., Kannagi, R., Dohi, T.: DNA hypermethylation contributes to incomplete synthesis of carbohydrate determinants in gastrointestinal cancer. Gastroenterology 135, 142–151 (2008)
Cheng, J.C., Yoo, C.B., Weisenberger, D.J., Chuang, J., Wozniak, C., Liang, G., Marquez, V.E., Greer, S., Orntoft, T.F., Thykjaer, T., Jones, P.A.: Preferential response of cancer cells to zebularine. Cancer Cell 6, 151–158 (2004)
Elias, A., Siegelin, M.D., Steinmüller, A., von Deimling, A., Lass, U., Korn, B., Mueller, W.: Epigenetic silencing of death receptor 4 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in gliomas. Clin. Cancer Res. 15, 5457–5465 (2009)
Bae, S.I., Cheriyath, V., Jacobs, B.S., Reu, F.J., Borden, E.C.: Reversal of methylation silencing of Apo2L/TRAIL receptor 1 (DR4) expression overcomes resistance of SK-MEL-3 and SK-MEL-28 melanoma cells to interferons (IFNs) or Apo2L/TRAIL. Oncogene 27, 490–498 (2008)
Horak, P., Pils, D., Haller, G., Pribill, I., Roessler, M., Tomek, S., Horvat, R., Zeillinger, R., Zielinski, C., Krainer, M.: Contribution of epigenetic silencing of tumor necrosis factor-related apoptosis inducing ligand receptor 1 (DR4) to TRAIL resistance and ovarian cancer. Mol. Cancer Res. 3, 335–343 (2005)
Fulda, S., Debatin, K.M.: 5-Aza-2′-deoxycytidine and IFN-gamma cooperate to sensitize for TRAIL-induced apoptosis by upregulating caspase-8. Oncogene 25, 5125–5133 (2006)
Hopkins-Donaldson, S., Ziegler, A., Kurtz, S., Bigosch, C., Kandioler, D., Ludwig, C., Zangemeister-Wittke, U., Stahel, R.: Silencing of death receptor and caspase-8 expression in small cell lung carcinoma cell lines and tumors by DNA methylation. Cell Death Differ. 10, 356–364 (2003)
Fulda, S., Küfer, M.U., Meyer, E., van Valen, F., Dockhorn-Dworniczak, B., Debatin, K.M.: Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene 20, 5865–5877 (2001)
Eggert, A., Grotzer, M.A., Zuzak, T.J., Wiewrodt, B.R., Ho, R., Ikegaki, N., Brodeur, G.M.: Resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in neuroblastoma cells correlates with a loss of caspase-8 expression. Cancer Res. 61, 1314–1319 (2001)
Grotzer, M.A., Eggert, A., Zuzak, T.J., Janss, A.J., Marwaha, S., Wiewrodt, B.R., Ikegaki, N., Brodeur, G.M., Phillips, P.C.: Resistance to TRAIL-induced apoptosis in primitive neuroectodermal brain tumor cells correlates with a loss of caspase-8 expression. Oncogene 19, 4604–4610 (2000)
Stresemann, C., Brueckner, B., Musch, T., Stopper, H., Lyko, F.: Functional diversity of DNA methyltransferase inhibitors in human cancer cell lines. Cancer Res. 66, 2794–2800 (2006)
Acknowledgments
This study was performed by a grant from the New Energy and Industrial Technology Development Organization (NEDO) as a part of the developing technology project on implementing sugar chain functions in Japan, a Grant-in-Aid for Scientific Research (A), No. 21249038, from the Japan Society for the Promotion of Science, a Grant-in-Aid for Cancer Research and Scientific Research on Priority Areas, No. 20014011, from the Ministry of Education, Science, and the Global COE program of Osaka University funded by the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 855 kb)
Rights and permissions
About this article
Cite this article
Moriwaki, K., Narisada, M., Imai, T. et al. The effect of epigenetic regulation of fucosylation on TRAIL-induced apoptosis. Glycoconj J 27, 649–659 (2010). https://doi.org/10.1007/s10719-010-9310-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-010-9310-5