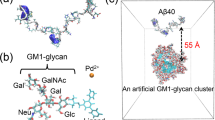
Abstract
Growing evidence has indicated that GM1 ganglioside specifically interacts with Amyloid β-peptide (Aβ) and thereby promotes Alzheimer’s disease-associated Aβ assembly. To characterize the conformation of Aβ bound to the ganglioside, we performed 920 MHz ultra-high field NMR analyses using isotopically labeled Aβ(1–40) in association with GM1 and lyso-GM1 micelles. Our NMR data revealed that (1) Aβ(1–40) forms discontinuous α-helices at the segments His14-Val24 and Ile31-Val36 upon binding to the gangliosidic micelles, leaving the remaining regions disordered, and (2) Aβ(1–40) lies on hydrophobic/hydrophilic interface of the ganglioside cluster exhibiting an up-and-down topological mode in which the two α-helices and the C-terminal dipeptide segment are in contact with the hydrophobic interior, whereas the remaining regions are exposed to the aqueous environment. These findings suggest that the ganglioside clusters serve as a unique platform for binding coupled with conformational transition of Aβ molecules, rendering their spatial rearrangements restricted to promote specific intermolecular interactions.




Similar content being viewed by others
Abbreviations
- Aβ:
-
Amyloid β-peptide
- AD:
-
Alzheimer’s disease
- CD:
-
Circular dichroism
- HSQC:
-
Heteronuclear single-quantum correlation
- NMR:
-
Nuclear magnetic resonance
- PG:
-
Phosphatidylglycerol
- TROSY:
-
Transverse relaxation-optimized spectroscopy
References
Selkoe, D.J.: Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 81, 741–766 (2001)
Hardy, J.A., Higgins, G.A.: Alzheimer’s disease: the amyloid cascade hypothesis. Science 256, 184–185 (1992)
Petkova, A.T., Yau, W.M., Tycko, R.: Experimental constraints on quaternary structure in Alzheimer's β-amyloid fibrils. Biochemistry 45, 498–512 (2006). doi:10.1021/bi051952q
Glabe, C.G.: Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol. Aging 27, 570–575 (2006). doi:10.1016/j.neurobiolaging.2005.04.017
Melchor, J.P., McVoy, L., Van Nostrand, W.E.: Charge alterations of E22 enhance the pathogenic properties of the amyloid β-protein. J. Neurochem. 74, 2209–2212 (2000). doi:10.1046/j.1471-4159.2000.0742209.x
Yanagisawa, K., Odaka, A., Suzuki, N., Ihara, Y.: GM1 ganglioside-bound amyloid β-protein (Aβ): A possible form of preamyloid in Alzheimer’s disease. Nat. Med. 1, 1062–1066 (1995). doi:10.1038/nm1095-1062
Matsuzaki, K.: Physicochemical interactions of amyloid β-peptide with lipid bilayers. Biochim. Biophys. Acta 1768, 1935–1942 (2007). doi:10.1016/j.bbamem.2007.02.009
Hayashi, H., Kimura, N., Yamaguchi, H., Hasegawa, K., Yokoseki, T., Shibata, M., Yamamoto, N., Michikawa, M., Yoshikawa, Y., Terao, K., Matsuzaki, K., Lemere, C.A., Selkoe, D.J., Naiki, H., Yanagisawa, K.: A seed for Alzheimer amyloid in the brain. J. Neurosci. 24, 4894–4902 (2004). doi:10.1523/JNEUROSCI.0861-04.2004
Yamamoto, N., Matsubara, E., Maeda, S., Minagawa, H., Takashima, A., Maruyama, W., Michikawa, M., Yanagisawa, K.: A ganglioside-induced toxic soluble Aβ assembly. Its enhanced formation from Aβ bearing the Arctic mutation. J. Biol. Chem. 282, 2646–2655 (2007). doi:10.1074/jbc.M606202200
Okada, T., Wakabayashi, M., Ikeda, K., Matsuzaki, K.: Formation of toxic fibrils of Alzheimer's amyloid β-protein-(1–40) by monosialoganglioside GM1, a neuronal membrane component. J. Mol. Biol. 371, 481–489 (2007). doi:10.1016/j.jmb.2007.05.069
Kakio, A., Nishimoto, S., Yanagisawa, K., Kozutsumi, Y., Matsuzaki, K.: Interactions of amyloid β-protein with various gangliosides in raft-like membranes: importance of GM1 ganglioside-bound form as an endogenous seed for Alzheimer amyloid. Biochemistry 41, 7385–7390 (2002). doi:10.1021/bi0255874
Williamson, M.P., Suzuki, Y., Bourne, N.T., Asakura, T.: Binding of amyloid β-peptide to ganglioside micelles is dependent on histidine-13. Biochem. J. 397, 483–490 (2006). doi:10.1042/BJ20060293
Kato, K., Sasakawa, H., Kamiya, Y., Utsumi, M., Nakano, M., Takahashi, N., Yamaguchi, Y.: 920 MHz ultra-high field NMR approaches to structural glycobiology. Biochim. Biophys. Acta 1780, 619–625 (2008)
Sasakawa, H., Sakata, E., Yamaguchi, Y., Masuda, M., Mori, T., Kurimoto, E., Iguchi, T., Hisanaga, S., Iwatsubo, T., Hasegawa, M., Kato, K.: Ultra-high field NMR studies of antibody binding and site-specific phosphorylation of α-synuclein. Biochem. Biophys. Res. Commun. 363, 795–799 (2007). doi:10.1016/j.bbrc.2007.09.048
Lee, E.K., Hwang, J.H., Shin, D.Y., Kim, D.I., Yoo, Y.J.: Production of recombinant amyloid-b peptide 42 as an ubiquitin extension. Protein Expr. Purif. 40, 183–189 (2005). doi:10.1016/j.pep.2004.12.014
Kakio, A., Nishimoto, S.I., Yanagisawa, K., Kozutsumi, Y., Matsuzaki, K.: Cholesterol-dependent formation of GM1 ganglioside-bound amyloid β-protein, an endogenous seed for Alzheimer amyloid. J. Biol. Chem. 276, 24985–24990 (2001). doi:10.1074/jbc.M100252200
Kurimoto, T., Asakura, K., Yamasaki, C., Nemoto, N.: MUSASHI: NMR pulse width determination method by nonlinear least square curve fitting. Chem. Lett. 34, 540–541 (2005). doi:10.1246/cl.2005.540
Pervushin, K., Riek, R., Wider, G., Wüthrich, K.: Attenuated T 2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc. Natl. Acad. Sci. USA 94, 12366–12371 (1997). doi:10.1073/pnas.94.23.12366
Clore, G.M., Gronenborn, A.M.: Multidimensional heteronuclear nuclear magnetic resonance of proteins. Methods Enzymol. 239, 349–363 (1994). doi:10.1016/S0076-6879(94)39013-4
Cornilescu, G., Delaglio, F., Bax, A.: Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J. Biomol. NMR 13, 289–302 (1999). doi:10.1023/A:1008392405740
Shimada, I.: NMR techniques for identifying the interface of a larger protein-protein complex: Cross-saturation and transferred cross-saturation experiments. Methods Enzymol. 394, 483–506 (2005). doi:10.1016/S0076-6879(05)94020-2
Spera, S., Ikura, M., Bax, A.: Measurement of the exchange rates of rapidly exchanging amide protons: Application to the study of calmodulin and its complex with a myosin light chain kinase fragment. J. Biomol. NMR 1, 155–165 (1991). doi:10.1007/BF01877227
Choo-Smith, L.P., Surewicz, W.K.: The interaction between Alzheimer amyloid β(1–40) peptide and ganglioside GM1-containing membranes. FEBS Lett. 402, 95–98 (1997). doi:10.1016/S0014-5793(96)01504-9
McLaurin, J., Chakrabartty, A.: Membrane disruption by Alzheimer β-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J. Biol. Chem. 271, 26482–26489 (1996). doi:10.1074/jbc.271.43.26482
Bradley, E.K., Thomason, J.F., Cohen, F.E., Kosen, P.A., Kuntz, I.D.: Studies of synthetic helical peptides using circular dichroism and nuclear magnetic resonance. J. Mol. Biol. 215, 607–622 (1990). doi:10.1016/S0022-2836(05)80172-X
Sticht, H., Bayer, P., Willbold, D., Dames, S., Hilbich, C., Beyreuther, K., Frank, R.W., Rosch, P.: Structure of amyloid A4-(1–40)-peptide of Alzheimer’s disease. Eur. J. Biochem. 233, 293–298 (1995). doi:10.1111/j.1432-1033.1995.293_1.x
Jarvet, J., Danielsson, J., Damberg, P., Oleszczuk, M., Gräslund, A.: Positioning of the Alzheimer Aβ(1–40) peptide in SDS micelles using NMR and paramagnetic probes. J. Biomol. NMR 39, 63–72 (2007). doi:10.1007/s10858-007-9176-4
Shao, H., Jao, S., Ma, K., Zagorski, M.G.: Solution structures of micelle-bound amyloid β-(1–40) and β-(1–42) peptides of Alzheimer’s disease. J. Mol. Biol. 285, 755–773 (1999). doi:10.1006/jmbi.1998.2348
Wahlström, A., Hugonin, L., Perálvarez-Marín, A., Jarvet, J., Gräslund, A.: Secondary structure conversions of Alzheimer's Aβ(1–40) peptide induced by membrane-mimicking detergents. FEBS J. 275, 5117–5128 (2008)
Sugase, K., Dyson, H.J., Wright, P.E.: Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature 447, 1021–1025 (2007). doi:10.1038/nature05858
Mandal, P.K., Pettegrew, J.W.: Alzheimer's disease: NMR studies of asialo (GM1) and trisialo (GT1b) ganglioside interactions with Aβ(1–40) peptide in a membrane mimic environment. Neurochem. Res. 29, 447–453 (2004). doi:10.1023/B:NERE.0000013750.80925.25
Iwahara, J., Zweckstetter, M., Clore, G.M.: NMR structural and kinetic characterization of a homeodomain diffusing and hopping on nonspecific DNA. Proc. Natl. Acad. Sci. USA 103, 15062–15067 (2006). doi:10.1073/pnas.0605868103
Acknowledgements
This work was supported in part by Nanotechnology Network Project and Grants in Aid for Scientific Research (18023032, 20023033) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by the Joint Studies Program of the Institute for Molecular Science, Japan. We thank Michiko Nakano for her help in the ultra-high field NMR measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Chemical shifts of 2H-, 13C-, and 15N-labeled Ab(1–40) bound to lyso-GM1a. a CaH chemical shifts were assigned for His6 (4.49 ppm), Val12 (4.35 ppm), His13 (4.49 ppm), and His14 (4.50 ppm) by using 13C- and 15N-labeled Ab(1–40) and also used for the TALOS calculation. b not detectable. (PDF 1.20 MB)
Supplementary Fig. 1
SupplementaryPlot of the molar ellipticity at 222 nm as a function of ganglioside-to-Ab(1–40) ratio. Lyso-GM1 (green), GM1 (blue) and GM1-containing liposomes composed of GM1/cholesterol/SM (40:30:30) (red). The values (mean + SD) were calculated from molar ellipticities at 222 nm in the three independent experiments. (PDF 1.20 MB)
Supplementary Fig. 2
1H–15N HSQC titration of 15N-labeled Ab(1–40) (0.2 mM) with GM1 in which Ab(1–40): GM1 concentration ratio of 1:0 (red), 1:2 (blue) and 1:30 (black). The spectra were recorded at the proton observation frequency of 500 MHz. The insets show close views of trajectories of the Phe4 and Tyr10 peaks boxed (magenta and cyan, respectively). (PDF 1.20 MB)
Supplementary Fig. 3
Plots of the intensity ratios of the backbone amide peaks of Ab(1–40) with on-resonance and off-resonance irradiation of the acyl (CH2) groups of GM1 (upper) and H2O (lower). Asterisk indicates the amino acid residue that did not exhibit observable peak in the spectrum due to severe broadening. Intensity ratios are the mean + SD of three independent experiments. (PDF 1.20 MB)
Rights and permissions
About this article
Cite this article
Utsumi, M., Yamaguchi, Y., Sasakawa, H. et al. Up-and-down topological mode of amyloid β-peptide lying on hydrophilic/hydrophobic interface of ganglioside clusters. Glycoconj J 26, 999–1006 (2009). https://doi.org/10.1007/s10719-008-9216-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-008-9216-7