Abstract
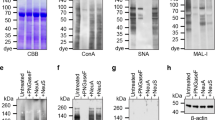
We reported previously that the dominant receptors of influenza A and B viruses, and human and murine respiroviruses, were sialylglycoproteins and gangliosides containing monosialo-lactosamine type I-and II-residues, such as sialic acid-α2-3(6)-Galβ1-3(4)-GlcNAcβ1-. In addition, the Siaα2-3Gal linkage was predominantly recognized by avian and horse influenza viruses, and human parainfluenza virus type 1 (hPIV-1), whereas the Siaα2-6Gal linkage was mainly recognized by human influenza viruses (Paulson JC in “The Receptors'' [Conn M Ed] 2, 131–219 (1985); Suzuki Y, Prog Lipid Res 33, 429–57 (1994); Ito T, J Virol 73, 6743–51 (2000); Suzuki Y, J Virol 74, 11825–31 (2000); Suzuki T, J. Virol 75, 4604–4613 (2001); Suzuki Y, Biol. Pharm. Bull. 28, 399–408 (2005)). To clarify the distribution of influenza virus receptors on the human bronchial epithelium cell surface, we investigated a primary culture of normal human bronchial epithelial (NHBE) cells using two types of lectin (MAA and SNA), which recognize sialyl linkages (α2-3 and α2-6), using fluorescence-activated cell-sorting analysis. The results showed that both α2-3- and α2-6-linked Sias were expressed on the surface of primary human bronchial epithelial cells. The cells infected by hPIV-1 bound to MAA, confirming that cells targeted by hPIV-1 have α2-3-linked oligosaccharides. We also compared the ability of hPIV-1 and human influenza A virus to infect primary human bronchial epithelial cells pre-treated with Siaα2-3Gal-specific sialidase from Salmonella typhimurium. No difference was observed in the number of sialidase pre-treated and non-treated cells infected with human influenza A virus, which binds to Siaα2-6Gal-linked oligosaccharides. By contrast, the number of cells infected with hPIV-1 decreased significantly upon sialidase treatment. Thus, cultured NHBE cells showed both α2-3-linked Sias recognized by hPIV-1 and avian influenza virus receptors, and α2-6-linked Sias recognized by human influenza virus receptors.
Similar content being viewed by others
References
Suzuki, Y., Ito, T., Suzuki, T., Holland, R.E., Chambers, T.M., Kiso, M., Ishida, H., Kawaoka, Y.: Sialic acid species as a determinant of the host range of influenza A viruses. J. Virol. 74, 11825–31 (2000)
Ito, T., Nelson, J., Couceiro, S.S., Kelm, S., Baum, G., Krauss, S., Castrucci, M.R., Donatelli, I., Kida, H., Paulson, J.C., Webster, R.G., Kawaoka, Y.: Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J. Virol. 72, 7367–73 (1998)
Rogers, G.N., Paulson, J.C.: Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 127, 361–73 (1983)
Rogers, G.N., Pritchett, T.J., Lane, J.L., Paulson, J.C.: Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: selection of receptor specific variants. Virology 131, 394–408 (1983)
Connor, R.J., Kawaoka, Y., Webster, R.G., Paulson, J.C.: Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology 205, 17–23 (1994)
Baum, L.G., Paulson, J.C.: Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta histochemica S(suppl. xl(s)) 35–8 (1990)
Couceiro, J.N., Paulson, J.C., Baum, L.G.: Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of the host cell in selection of hemagglutinin receptor specificity. Virus Research 29, 155–65 (1993)
Suzuki, T., Portner, A., Scroggs, R.A., Uchikawa, M., Koyama, N., Matsuo, K., Suzuki, Y., Takimoto, T.: Receptor specificities of human respiroviruses. J. Virol. 75, 4604–13 (2001)
Mercer, R.R., Russell, M.L., Roggli, V.L., Crapo, J.D.: Cell number and distribution in human and rat airways. Am. J. Respir. Cell. Mol. Biol. 10, 613–24 (1994)
Gorman, W.L., Pridgen, C., Portner, A.: Glycosylation of the hemagglutinin- neuraminidase glycoprotein of human parainfluenza virus type 1 affects its functional but not its antigenic properties. Virology 183, 83–90 (1991)
Portner, A., Scroggs, R.A., Metzger, D.W.: Distinct functions of antigenic sites of the HN glycoprotein of Sendai virus. Virology 158, 61–8 (1987)
Suzuki, Y., Matsunaga, M., Matsumoto, M.: N-Acetylneura- minyllactosylceramide, GM3-NeuAc, a new influenza A virus receptor which mediates the adsorption-fusion process of viral infection. J. Biol. Chem. 260, 1362–5 (1985)
Suzuki, T., Sometani, A., Yamazaki, Y., Horiike, G., Mizutani, Y., Masuda, H., Yamada, M., Tahara, H., Xu, G., Miyamoto, D., Oku, N., Okada, S., Kiso, M., Hasegawa, A., Ito, T., Kawaoka, Y., Suzuki, Y.: Sulphatide binds to human and animal influenza A viruses, and inhibits the viral infection. Biochem. J. 318, 389–93 (1996)
Suzuki, Y., Nakao, T., Ito, T., Watanabe, N., Toda, Y., Xu, G.Y., Suzuki, T., Kobayashi, T., Kimura, Y., Yamada, A., Sugawara, K., Nishimura, H., Kitame, F., Nakamura, K., Deya, E., Kiso, M., Hasegawa, A.: Structural determination of gangliosides that bind to influenza A, B, and C viruses by an improved binding assay: strain-specific receptor epitopes in sialo-sugar chains. Virology 189, 121–31 (1992)
Shibuya, N., Goldstein, I.F., Broekaert, W.F., Nsimba-Lubake, M., Peeters, B., Peumans, W.J.: The elderberry (Sambucus nigra L.) bark lectin recognizes the NeuAc(α2,6)Gal/GalNac sequence. J. Biol. Chem. 262, 1596–601 (1987)
Wang, W.C., Cummings, R.D.: The immobilized leukoagglutinins from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid linked α-2,3 linked to the penultimate galactose residues. J. Biol. Chem. 263, 4576–85 (1988)
Hoyer, L.L., Roggentin, P., Schauer, R., Vimr, E.R.: Purification and properties of cloned Salmonella typhimurium LT2 sialidase with virus-typical kinetic preference for sialyl α2→3 linkages. J Biochem 110, 462–7 (1991)
Ito, T., Suzuki, Y., Suzuki, T., Tanaka, A., Horimoto, T., Wells, K., Kida, H., Otsuki, K., Kiso, M., Ishida, H., Kawaoka, Y.: Recognition of N-glycolylneuraminic acid linked to galactose by the alpha2,3 linkage is associated with intestinal replication of influenza A virus in ducks. J. Virol. 73, 6743–51 (2000)
Ito, T., Suzuki, Y., Takada, A., Kawamoto, A., Otsuki, K., Masuda, H., Suzuki, T., Kida, H., Kawaoka, Y.: Differences in sialic acid-galactose linkages in the chicken egg amnion and allantois influence human influenza virus receptor specificity and variant selection. J. Virol. 71, 3357–62 (1997)
Pyan-Poirier, K., Suzuki, Y., Bean, W.J., Kobasa, D., Takada, A., Ito, T., Kawaoka, Y.: Changes in H3 influenza A virus receptor specificity during replication in humans. Virus. Res. 56, 169–76 (1998)
Snyder, M.H., Clements, M.L., Borde, D.D., Maassab, H.F., Murphy, B.R.: Attenuation of wild-type human influenza A virus by acquisition of the PA polymerase and matrix protein genes of influenza A/Ann Arbor/6/60 cold-adapted donor virus. J. Clin. Microbiol. 22, 719–25 (1987)
Ito, T., Couceiro, J.N.S.S., Kelm, S., Baum, S., Krauss, S., Castrucci, M.R., Donatelli, I., Kida, H., Paulson, J.C., Webster, R.G., Kawaoka, Y.: Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J. Virol. 72, 7367–73 (1998)
World Health Organization, Influenza activity, Morbid Mortal Wkly Rep. 46, 1204 (1997)
Subbarao, K., Klimov, A., Katz, J., Regnery, H., Lim, W., Hall, H., Perdue, M., Swayne, D., Bender, C., Huang, J., Hemphill, M., Rowe, T., Shaw, M., Xu, X., Fukuda, K., Cox, N.: Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 279, 393–6 (1998)
Claas, E.C., Osterhaus, A.D., van Beek, R., De Jong, J.C., Rimmelzwaan, G.F., Senne, D.A., Krauss, S., Shortridge, K.F., Webster, R.G.: Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351, 472–77 (1998)
Cauthern, A.N., Swayne, D.E., Schultz-Cherry, S., Pergue, M.L., Suarez, D.L.: Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans. J. Virol. 74, 6592–99 (2000)
Guan, Y., Poon, L.L.M., Cheung, C.Y., Ellis, T.M., Lim, W., Lipatov, A.S., Chan, K.H., Sturm-Ramirez, K.M., Cheung, C.L., Leung, Y.H.C., Yuen, K.Y., Webster, R.G., Peiris, J.S.M.: H5N1 influenza: A protean pandemic threat. Proc. Natl. Acad. Sci. 101, 8156–61 (2004)
Matrosovich, M., Zhou, N., Kawaoka, Y., Webster, R.G.: The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J. Virol. 73, 1146–55 (1999)
Lipatov, A.S., Govorkova, E.A., Webby, R.J., Ozaki, H., Peiris, M., Guan, Y., Poon, L.: Webster RG (Minireview), Influenza: Emergence and control. J. Virol. 78, 8951–9, (2004)
Matrovich, M.N., Kraus, S., Webster, R.G.: H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology 281, 156–62 (2001)
Saito, T., Lim, W., Suzuki, T., Suzuki, Y., Kida, H., Nishimura, S.-I., Tashiro, M.: Characterization of a human H9N2 influenza virus isolated in Hong Kong. Vaccine 20, 125–33 (2001)
Matrosovich, M.N., Matrosovich, T.Y., Gray, T., Roberts, N.A., Klenk, H.D.: Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. 101, 4620–24 (2004)
Beare, A.S., Webster, R.G.: Replication of avian influenza viruses in humans. Arch. Virol. 119, 37–42 (1991)
Murphy, B.R., Hinshaw, V.S., Sly, D.L., London, W.T., Hosier, N.T., Wood, F.T., Webster, R.G., Chanock, R.M.: Virulence of avian influenza A viruses for squirrel monkeys. Infect. Immun. 37, 1119–26 (1982)
Snyder, M.H., Buckler-White, A.J., London, W.T., Tierney, E.L., Murphy, B.R.: The avian influenza virus nucleoprotein gene and a specific constellation of avian and human virus polymerase genes each specify attenuation of avian-human influenza A/Pintail/79 reassortant viruses for monkeys. J. Virol. 61, 2857–63 (1987)
Hinshaw, V.S., Webster, R.G., Naeve, C.W., Murphy, B.R.: Altered tissue tropism of human-avian reassortant influenza viruses. Virology 128, 260–3 (1983)
Kida, H., Yanagawa, R., Matsuoka, Y.: Duck influenza lacking evidence of disease signs and immune response. Infect Immun 30, 547–53 (1980)
Webster, R.G., Yakhno, M., Hinshaw, V.S., Bean, W.J., Murti, K.G.: Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology 842, 268–78 (1978)
Suzuki, Y.: (Review), Sialobiology of influenza – molecular mechanism of host range variation of influenza viruses. Biol. Pharm. Bull. submitted (2005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kogure, T., Suzuki, T., Takahashi, T. et al. Human trachea primary epithelial cells express both sialyl(α2-3)Gal receptor for human parainfluenza virus type 1 and avian influenza viruses, and sialyl(α2-6)Gal receptor for human influenza viruses. Glycoconj J 23, 101–106 (2006). https://doi.org/10.1007/s10719-006-5442-z
Issue Date:
DOI: https://doi.org/10.1007/s10719-006-5442-z