Abstract
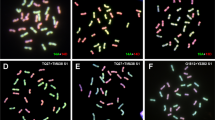
Polyploids are defined as either autopolyploids or allopolyploids, depending on their mode of origin and/or chromosome pairing behaviour. Autopolyploids have chromosome sets that are the result of the duplication or combination of related genomes (e.g., AAAA), while allopolyploids result from the combination of sets of chromosomes from two or more different taxa (e.g., AABB, AABBCC). Allopolyploids are expected to show preferential pairing of homologous chromosomes from within each parental sub-genome, leading to disomic inheritance. In contrast, autopolyploids are expected to show random pairing of chromosomes (non-preferential pairing), potentially leading to polysomic inheritance. The two main cultivated taxa of Actinidia (kiwifruit) are A. chinensis (2x and 4x) and A. chinensis var. deliciosa (6x). There is debate whether A. chinensis var. deliciosa is an autopolyploid derived solely from A. chinensis or whether it is an allopolyploid derived from A. chinensis and one or two other Actinidia taxa. To investigate whether preferential or non-preferential chromosome pairing occurs in A. chinensis var. deliciosa, the inheritance of microsatellite alleles was analysed in the tetraploid progeny of a cross between A. chinensis var. deliciosa and the distantly related Actinidia eriantha Benth. (2x). The frequencies of inherited microsatellite allelic combinations in the hybrids suggested that non-preferential chromosome pairing had occurred in the A. chinensis var. deliciosa parent. Meiotic chromosome analysis showed predominantly bivalent formation in A. chinensis var. deliciosa, but a low frequency of quadrivalent chromosome formations was observed (1 observed in 20 pollen mother cells).

Similar content being viewed by others
References
Andras SC, Hartman TPV, Marshall JA, Marchant R, Power JB, Cocking EC, Davey MR (1999) A dropspreading technique to produce cytoplasm-free mitotic preparations from plants with small chromosomes. Chromosome Res 7:641–647
Atkinson RG, Cipriani G, Whittaker DJ, Gardner RC (1997) The allopolyploid origin of kiwifruit, Actinidia deliciosa (Actinidiaceae). Plant Syst Evol 205:111–124
Chester M, Gallagher JP, Symonds VV, Cruz da Silva AV, Mavrodiev EV, Leitch AR, Soltis PS, Soltis DE (2012) Extensive chromosomal variation in a recently formed natural allopolyploid species, Tragopogon miscellus (Asteraceae). Proc Natl Acad Sci USA 109:1176–1181
Crawford DJ, Smith EB (1984) Allozyme divergence and intraspecific variation in Coreopsis grandiflora (Compositae). Sys Bot 9:219–225
Crowhurst RN, Lints R, Atkinson RG, Gardner RC (1990) Restriction fragment length polymorphisms in the genus Actinidia (Actinidiaceae). Plant Syst Evol 172:193–203
Cui L, Wall PK, Leebens-Mack JH, Lindsay BG, Douglas E, Soltis DE, Doyle JJ, Soltis PS, Carlson JE, Arumuganathan K, Barakat A, Albert VA, Hong Ma H, dePamphilis CW (2006) Widespread genome duplications throughout the history of flowering plants. Genome Res 16:738–749
Datson PM, Ferguson AR (2011) Kiwifruit (Actinidia). In: Kole CD (ed) Wild crop relatives: genomic and breeding resources. Tropical and subtropical fruits. Springer, Heidelberg, pp 1–20
Ferguson AR, Huang H-W (2007) Genetic resources of kiwifruit: domestication and breeding. Hort Rev 33:1–121
Fjellstrom RG, Beuselinck PR, Steiner JJ (2001) RFLP marker analysis supports tetrasomic inheritance in Lotus corniculatus L. Theor Appl Genet 102:718–725
Fraser LG, Tsang GK, Datson PM, De Silva HN, Harvey CF, Gill GP, Crowhurst RN, McNeilage MA (2009) A gene-rich linkage map in the dioecious species Actinidia chinensis (kiwifruit) reveals putative X/Y sex-determining chromosomes. BMC Genome 10:102
He Z-C, Wang S-M, Huang H, Huang H-Q (1998) Study on chromosome numbers of 6 species and 1 variety in Actinidia Lindl. J Wuhan Bot Res 16:299–301
Henegariu O, Heerema NA, Wright LL, Bray-Ward P, Ward DC, Vance GH (2001) Improvements in cytogenetic slide preparation: controlled chromosome spreading, chemical aging and gradual denaturing. Cytometry 43:101–109
Huang H-W, Dane F, Wang Z–Z, Jiang Z-W, Huang R-H, Wang S-M (1997) Isozyme inheritance and variation in Actinidia. Hered 78:328–336
Jones GH (1994) Meiosis in autopolyploid Crepis capillaris. III. Comparison of triploids and tetraploids: evidence for non independence of autonomous pairing sites. Hered 73:215–219
Li J-Q, Huang H-W, Sang T (2002) Molecular phylogeny and infrageneric classification of Actinidia (Actinidiaceae). Syst Bot 27:408–415
Li J-Q, Li X-W, Soejarto DD (2007a) Actinidiaceae. In: Wu Z-Y, Raven PH, Hong D-Y (eds) Flora of China, Hippocastanaceae through Theaceae, vol 12. Science Press, Beijing, Missouri Botanical Garden Press, St Louis, MO, USA, pp 334–360
Li X-W, Li J-Q, Soejarto DD (2007b) New synonyms in Actinidiaceae from China. Acta Phylotaxon Sin 45:633–660
Li D-W, Zhong C-H, Liu Y-F, Huang H-W (2010) Correlation between ploidy level and fruit characters of the main kiwifruit cultivars in China: implication for selection and improvement. NZ J Crop Hort Sci 38:137–145
Liang C-F, Ferguson AR (1984) Emendation of the Latin name of Actinidia chinensis Planch. var. hispida C.F.Liang. Guihaia 4:181–182
McNeilage MA, Considine JA (1989) Chromosome studies in some Actinidia taxa and implications for breeding. NZ J Bot 27:71–81
Qu L, Hancock JF, Whallon JH (1998) Evolution in an autopolyploid group displaying predominantly bivalent pairing at meiosis: genomic similarity of diploid Vaccinium darrowi and autotetraploid V. corymbosum (Ericaceae). Am J Bot 85:698–703
Ramsey J, Schemske DW (2002) Neopolyploidy in flowering plants. Annu Rev Ecol Syst 33:589–639
Ripol MI, Churchill GA, da Silva JA, Sorrells M (1999) Statistical aspects of genetic mapping in autopolyploids. Gene 235:31–41
Samuel R, Pinsker W, Ehrendorfer F (1990) Allozyme polymorphism in diploid and polyploid populations of Galium. Hered 65:369–378
Soltis DE, Rieseberg LH (1986) Autopolyploidy in Tolmiea menziesii (Saxifragaceae): genetic insights from enzyme electrophoresis. Am J of Bot 73:310–318
Soltis DE, Soltis PS (1993) Molecular data and the dynamic nature of polyploidy. Crit Rev Plant Sci 12:243–273
Testolin R, Ferguson AR (1997) Isozyme polymorphism in the genus Actinidia and the origin of the kiwifruit genome. Syst Bot 22:685–700
Watanabe K, Takahashi B, Shirato K (1990) Chromosome numbers in kiwifruit (Actinidia deliciosa) and related species. J Japan Soc Hort Sci 58:835–840
Wood TE, Takebayashi N, Barker MS, Mayrose I, Greenspoon PB, Rieseberg LH (2009) The frequency of polyploid speciation in vascular plants. Proc Natl Acad Sci USA 106:13875–13879
Yan G-J, Ferguson AR, McNeilage MA, Murray BG (1997) Numerically unreduced (2n) gametes and sexual polyploidization in Actinidia. Euphytica 96:267–272
Zhen Y-Q, Li Z–Z, Huang H-W, Wang Y (2004) Molecular characterization of kiwifruit (Actinidia) cultivars and selections using SSR markers. J Am Soc Hort Sci 129:374–382
Acknowledgments
We would like to thank Dr. Alan Seal for providing plants, Dr. Nihal De Silva, Dr. Alan Seal, Dr. Samantha Baldwin, Dr. Anne Gunson and Dr. Ross Ferguson for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mertten, D., Tsang, G.K., Manako, K.I. et al. Meiotic chromosome pairing in Actinidia chinensis var. deliciosa . Genetica 140, 455–462 (2012). https://doi.org/10.1007/s10709-012-9693-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-012-9693-2