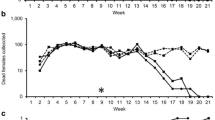
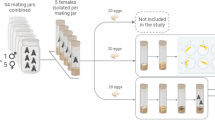
Abstract
Insect transgenesis is continuously being improved to increase the efficacy of population suppression and replacement strategies directed to the control of insect species of economic and sanitary interest. An essential prerequisite for the success of both pest control applications is that the fitness of the transformant individuals is not impaired, so that, once released in the field, they can efficiently compete with or even out-compete their wild-type counterparts for matings in order to reduce the population size, or to spread desirable genes into the target population. Recent research has shown that the production of fit and competitive transformants can now be achieved and that transgenes may not necessarily confer a fitness cost. In this article we review the most recent published results of the fitness assessment of different transgenic insect lines and underline the necessity to fulfill key requirements of ecological safety. Fitness evaluation studies performed in field cages and medium/large-scale rearing will validate the present encouraging laboratory results, giving an indication of the performance of the transgenic insect genotype after release in pest control programmes.


Similar content being viewed by others
References
Abraham EG, Donnelly-Doman M, Fujioka H, Ghosh A, Moreira L, Jacobs-Lorena M (2005) Driving midgut-specific expression and secretion of a foreign protein in transgenic mosquitoes with AgAper1 regulatory elements. Insect Mol Biol 14:271–279
Ahmed AM, Hurd H (2006) Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicular apoptosis. Microbes Infect 8:308–315
Allen ML, Scholl PJ (2005) Quality of transgenic laboratory strains of Cochliomyia hominivorax (Diptera: Calliphoridae). J Econ Entomol 98:2301–2306
Allen ML, Handler AM, Berkebile DR, Skoda SR (2004a) PiggyBac transformation of the new world screwworm, Cochliomyia hominivorax, produces multiple distinct mutant strains. Med Vet Entomol 18:1–9
Allen ML, Berkebile DR, Skoda SR (2004b) Postlarval fitness of transgenic strains of Cochliomyia hominivorax (Diptera: Calliphoridae). J Econ Entomol 97:1181–1185
Alphey L (2007) Engineering insects for the sterile insect technique. In: Vreysen M, Robinson A, Hendrichs J (eds) Area-wide control of insect pests: from research to field implementation. Springer, Dordrecht, pp 51–60
Alphey L, Andreasen M (2002) Dominant lethality and insect population control. Mol Biochem Parasitol 121:173–178
Alphey L, Beard B, Billingsley P, Coetzee M, Crisanti A, Curtis CF, Eggleston P, Godfray C, Hemingway J, Jacobs-Lorena M, James A, Kafatos F, Mukwaya L, Paton M, Powell J, Schneider W, Scott T, Sine B, Sinden R, Sinkins S, Spielman A, Touré Y, Collins F (2002) Malaria control with genetically modified vectors. Science 298:119–121
Amenya DA, Bonizzoni M, Isaacs AT, Jasinskiene N, Chen H, Marinotti O, Yan G, James AA (2010) Comparative fitness assessment of Anopheles stephensi transgenic lines receptive to site-specific integration. Insect Mol Biol 19:263–269
Atkinson PW, Pinkerton AC, O’Brochta DA (2001) Genetic transformation systems in insects. Annu Rev Entomol 46:317–346
Bellini R, Calvitti M, Medici A, Carrieri M, Celli G, Maini S (2007) Use of the sterile insect technique against Aedes albopictus in Italy: first results of a pilot trial. In: Vreysen MB, Robinson AS, Hendrichs J (eds) Area-wide control of insect pests. Springer, Dordrecht, pp 505–515
Bellows TS, Paine TD, Gould JR, Bezark LG, Ball J (1992) Biological control of ash whitefly: a success in progress. Calif Agric 46:124–128
Benedict MQ, Robinson AS (2003) The first releases of transgenic mosquitoes: an argument for the sterile insect technique. Trends Parasitol 19:349–355
Benedict MQ, Robinson AS (2008) Impact of technological improvements on traditional control strategies. In: Aksoy S (ed) Transgenesis and management of vector-borne diseases. Landes Bioscience, New York, pp 84–90
Bergmann A, Agapite J, McCall K, Steller H (1998) The Drosophila gene hid is a direct molecular target of Ras-dependent survival signaling. Cell 95:331–341
Bloem KA, Bloem S, Carpenter JE (2005) Impact of moth suppression/eradication programmes using the sterile insect technique or inherited sterility. In: Dyck VA, Hendrichs J, Robinson AS (eds) Sterile insect technique. Principles and practice in area-wide integrated pest management. Springer, Dordrecht, pp 677–700
Boëte C (2005) Malaria parasites in mosquitoes: laboratory models, evolutionary temptation and the real world. Trends Parasitol 21:445–447
Boete C, Koella JC (2002) A theoretical approach to predicting the success of genetic manipulation of malaria mosquitoes in malaria control. Malar J 1:3
Catteruccia F, Nolan T, Loukeris TG, Blass C, Savakis C, Kafatos FC, Crisanti A (2000) Stable germline transformation of the malaria mosquito Anopheles stephensi. Nature 405:959–962
Catteruccia F, Godfray HCJ, Crisanti A (2003) Impact of genetic manipulation on the fitness of Anopheles stephensi mosquitoes. Science 299:1225–1227
Catteruccia F, Benton JP, Crisanti A (2005) An Anopheles transgenic sexing strain for vector control. Nat Biotechnol 23:1414–1417
Condon KC, Condon GC, Dafa’alla TH, Forrester OT, Phillips CE, Scaife S, Alphey L (2007a) Germ-line transformation of the Mexican fruit fly. Insect Mol Biol 16:573–580
Condon KC, Condon GC, Dafa’alla TH, Fu G, Phillips CE, Jin L, Gong P, Alphey L (2007b) Genetic sexing through the use of Y-linked transgenes. Insect Biochem Mol Biol 37:1168–1176
Davis S, Bax N, Grewe P (2001) Engineered underdominance allows efficient and economical introgression of traits into pest populations. J Theor Biol 212:83–98
Enkerlin WR (2005) Impact of fruit fly control programmes using the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS (eds) Sterile insect technique. Principles and practice in area-wide integrated pest management. Springer, Dordrecht, pp 651–676
Franz AW, Sanchez-Vargas I, Adelman ZN, Blair CD, Beaty BJ, James AA, Olson KE (2006) Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc Natl Acad Sci USA 103:4198–4203
Fu G, Condon KC, Epton MJ, Gong P, Jin L, Condon GC, Morrison NI, Dafa’alla TH, Alphey L (2007) Female-specific insect lethality engineered using alternative splicing. Nat Biotechnol 25:353–357
Fu G, Lees RS, Nimmo D, Aw D, Jin L, Gray P, Berendonk TU, White-Cooper H, Scaife S, Kim Phuc H, Marinotti O, Jasinskiene N, James AA, Alphey L (2010) Female-specific flightless phenotype for mosquito control. Proc Natl Acad Sci USA 107:4550–4554
Gong P, Epton MJ, Fu G, Scaife S, Hiscox A, Condon KC, Condon GC, Morrison NI, Kelly DW, Dafa’alla TH, Coleman PG, Alphey L (2005) A dominant lethal genetic system for autocidal control of the Mediterranean fruit fly. Nat Biotechnol 23:453–456
Grossman GL, Rafferty CS, Clayton JR, Stevens TK, Mukabayire O, Benedict MQ (2001) Germline transformation of the malaria vector Anopheles gambiae, with the piggyBac transposable element. Insect Mol Biol 10:597–604
Hahn MW, Nuzhdin SV (2004) The fixation of malaria refractoriness in mosquitoes. Curr Biol 14:264–265
Handler AM (2002) Prospects for using genetic transformation for improved SIT and new biocontrol methods. Genetica 116:137–149
Handler AM, Harrell RA II (2001) Transformation of the Caribbean fruit fly, Anastrepha suspensa, with a piggyBac vector marked with polyubiquitin-regulated GFP. Insect Biochem Mol Biol 31:199–205
Handler AM, McCombs SD (2000) The piggyBac transposon mediates germ-line transformation in the Oriental fruit fly and closely related elements exist in its genome. Insect Mol Biol 9:605–612
Handler AM, O’Brochta DA (2005) Transposable elements for insect transformation. In: Gilbert LI, Iatrou K, Gill S (eds) Comprehensive insect physiology, biochemistry, pharmacology, and molecular biology. Elsevier Ltd, Oxford, pp 437–474
Handler AM, McCombs SD, Fraser MJ, Saul SH (1998) The lepidopteran transposon vector, piggyBac, mediates germ-line transformation in the Mediterranean fruit fly. Proc Natl Acad Sci USA 95:7520–7525
Handler AM, Allen ML, Skoda SR (2009) Development and utilization of transgenic new world screwworm, Cochliomyia hominivorax. Med Vet Entomol 23(1):98–105
Heinrich J, Scott M (2000) A repressible female-specific lethal genetic system for making transgenic insect strains suitable for a sterile-release program. Proc Natl Acad Sci USA 97:8229–8232
Hendrichs J (2000) Use of the sterile insect technique against key insect pests. Sustainable Dev Int 2:75–79
Horn C, Wimmer EA (2003) A transgene-based, embryo-specific lethality system for insect pest management. Nat Biotechnol 21:64–70
Huang Y, Magor K, Lloyd AL, Gould F (2007) Introducing desirable transgenes into insect populations using Y-linked meiotic drive—a theoretical assessment. Evolution 61:717–726
Hurd H, Taylor PJ, Adams D, Underhill A, Eggleston P (2005) Evaluating the costs of mosquito resistance to malaria parasites. Evol Int J Org Evol 59:2560–2572
Irvin N, Hoddle MS, O’Brochta DA, Carey B, Atkinson PW (2004) Assessing fitness costs for transgenic Aedes aegypti expressing the GFP marker and transposase genes. Proc Natl Acad Sci USA 101:891–896
Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M (2002) Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature 417:452–455
James AA (2005) Gene drive systems in mosquitoes: rules of the road. Trends Parasitol 21:64–67
Jordan IK, Matyunina LV, McDonald JF (1999) Evidence for the recent horizontal transfer of long terminal repeat retrotransposon. Proc Natl Acad Sci USA 96:12621–12625
Kidwell MG (1992a) Horizontal transfer. Curr Opin Genet Dev 2:868–873
Kidwell MG (1992b) Horizontal transfer of P elements and other short inverted repeat transposons. Genetica 86:275–286
Kim W, Koo H, Richman AM, Seeley D, Vizioli J, Klocko AD, O’Brochta DA (2004) Ectopic expression of a cecropin transgene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): effects on susceptibility to Plasmodium. J Med Entomol 41:447–455
Klassen W, Curtis CF (2005) History of the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS (eds) Sterile insect technique. Principles and practice in area-wide integrated pest management. Springer, Dordrecht, pp 3–36
Knipling E (1955) Possibilities of insect control or eradication through the use of sexually sterile males. J Econ Entomol 48:459–462
Knols BG, Njiru BN, Mathenge EM, Mukabana WR, Beier JC, Killeen GF (2002) MalariaSphere: a greenhouse-enclosed simulation of a natural Anopheles gambiae (Diptera: Culicidae) ecosystem in western Kenya. Malar J 1:19
Koukidou M, Klinakis A, Reboulakis C, Zagoraiou L, Tavernarakis N, Livadaras I, Economopoulos A, Savakis C (2006) Germ line transformation of the olive fly Bactrocera oleae using a versatile transgenesis marker. Insect Mol Biol 15:95–103
Lambrechts L, Koella JC, Boëte C (2007) Can transgenic mosquitoes afford the fitness cost? Trends Parasitol 24:4–7
Landahl JT, Root RB (1969) Differences in the life tables of tropical and temperate milkweed bugs, genus oncopeltus (Hemiptera:Lygaeidae). Ecology 50:734–737
Li C, Marrelli MT, Yan G, Jacobs-Lorena M (2008) Fitness of transgenic Anopheles stephensi mosquitoes expressing the SM1 peptide under the control of a vitellogenin promoter. J Hered 99:275–282
Liu HS, Jan MS, Chou CK, Chen PH, Ke NJ (1999) Is green fluorescent protein toxic to the living cells? Biochem Biophys Res Commun 260:712–717
Loukeris TG, Livadaras I, Arcà B, Zabalou S, Savakis C (1995) Gene transfer into the medfly, Ceratitis capitata, with a Drosophila hydei transposable element. Science 270:2002–2005
Mackay TF (1989) Transposable elements and fitness in Drosophila melanogaster. Genome 31:284–295
Markstein M, Pitsouli C, Villalta C, Celniker SE, Perrimon N (2008) Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat Genet 40:476–483
Marrelli MT, Moreira CK, Kelley D, Alphey L, Jacobs-Lorena M (2006) Mosquito transgenesis: what is the fitness cost? Trends Parasitol 22:197–202
Marrelli MT, Li C, Rasgon JL, Jacobs-Lorena M (2007) Transgenic malaria-resistant mosquitoes have a fitness advantage when feeding on Plasmodium-infected blood. Proc Natl Acad Sci USA 104:5580–5583
Maynard Smith J, Haigh J (1974) The hitch-hiking effect of a favourable gene. Genet Res 23:23–35
Michel K, Stamenova A, Pinkerton AC, Franz G, Robinson AS, Gariou-Papalexiou A, Zacharopoulou A, O’Brochta DA, Atkinson PW (2001) Hermes-mediated germ-line transformation of the Mediterranean fruit fly Ceratitis capitata. Insect Mol Biol 10:155–162
Moreira LA, Ito J, Ghosh A, Devenport M, Zieler H, Abraham EG, Crisanti A, Nolan T, Catteruccia F, Jacobs-Lorena M (2002) Bee venom phospholipase inhibits malaria parasite development in transgenic mosquitoes. J Biol Chem 277:40839–40843
Moreira LA, Wang J, Collins FH, Jacobs-Lorena M (2004) Fitness of Anopheline mosquitoes expressing transgenes that inhibit Plasmodium development. Genetics 166:1337–1341
Mori A, Chadee DD, Graham DH, Severson DW (2004) Reinvestigation of an endogenous meiotic drive system in the mosquito, Aedes aegypti (Diptera: Culicidae). J Med Entomol 41:1027–1033
Nimmo DD, Alphey L, Meredith JM, Eggleston P (2006) High efficiency site-specific genetic engineering of the mosquito genome. Insect Mol Biol 15:129–136
O’Brochta DA, Sethuraman N, Wilson R, Hice RH, Pinkerton AC, Levesque CS, Bideshi DK, Jasinskiene N, Coates CJ, James AA, Lehane MJ, Atkinson PW (2003) Gene vector and transposable element behavior in mosquitoes. J Exp Biol 206:3823–3834
Perera OP, Harrell IR, Handler AM (2002) Germ-line transformation of the South American malaria vector, Anopheles albimanus, with a piggyBac/EGFP transposon vector is routine and highly efficient. Insect Mol Biol 11:291–297
Phuc HK, Andreasen MH, Burton RS, Vass C, Epton MJ, Pape G, Fu G, Condon KC, Scaife S, Donnelly CA, Coleman PG, White-Cooper H, Alphey L (2007) Late-acting dominant lethal genetic systems and mosquito control. BMC Biol 5:11
Rasgon JL, Gould F (2005) Transposable element insertion location bias and the dynamics of gene drive in mosquito populations. Insect Mol Biol 14:493–500
Rasgon JL, Scott TW (2003) Wolbachia and cytoplasmic incompatibility in the California Culex pipiens mosquito species complex: parameter estimates and infection dynamics in natural populations. Genetics 165:2029–2038
Robertson HM, Lampe DJ (1995) Recent horizontal transfer of a mariner transposable element among and between Diptera and Neuroptera. Mol Biol Evol 12:850–862
Robinson AS, Vreysen MJB, Hendrichs J, Feldmann U (2009) Enabling technologies to improve area-wide integrated pest management programmes for the control of screwworms. Med Vet Entomol 23(Suppl 1):1–7
Rodrigues FG, Santos MN, de Carvalho TX, Rocha BC, Riehle MA, Pimenta PF, Abraham EG, Jacobs-Lorena M, Alves de Brito CF, Moreira LA (2008) Expression of a mutated phospholipase A2 in transgenic Aedes fluviatilis mosquitoes impacts Plasmodium gallinaceum development. Insect Mol Biol 17:175–183
Santos MN, Nogueira PM, Dias FBS, Valle D, Moreira LA (2010) Fitness aspects of transgenic Aedes fluviatilis mosquitoes expressing a Plasmodium-blocking molecule. Transgenic Res. doi:10.1007/s11248-010-9375-8
Schetelig MF, Caceres C, Zacharopoulou A, Franz G, Wimmer EA (2009a) Conditional embryonic lethality to improve the sterile insect technique in Ceratitis capitata (Diptera:Tephritidae). BMC Biol 7:4
Schetelig MF, Scolari F, Handler AM, Kittelmann S, Gasperi G, Wimmer EA (2009b) Site-specific recombination for the modification of transgenic strains of the Mediterranean fruit fly Ceratitis capitata. Proc Natl Acad Sci USA 106:18171–18176
Schliekelman P, Gould F (2000) Pest control by the release of insects carrying a female-killing allele on multiple loci. J Econ Entomol 93:1566–1579
Scolari F, Schetelig MF, Gabrieli P, Siciliano P, Gomulski LM, Karam N, Wimmer EA, Malacrida AR, Gasperi G (2008a) Insect transgenesis applied to tephritid pest control. J Appl Entomol 132:820–831
Scolari F, Schetelig MF, Bertin B, Malacrida AR, Gasperi G, Wimmer EA (2008b) Fluorescent sperm marking to improve the fight against the pest insect Ceratitis capitata (Wiedemann; Diptera: Tephritidae). N Biotechnol 25:76–84
Scott TW, Takken W, Knols BGJ, Boëte C (2002) The ecology of genetically modified mosquitoes. Science 298:117–119
Scott TW, Rasgon JL, Black WC IV, Gould F (2005) Fitness studies: developing a consensus methodology. In: Knols BGJ, Louis C (eds) Strategic plan to bridge laboratory and field research in disease vector control. Frontis, Dordrecht, pp 171–181
Simmons GM (1992) Horizontal transfer of hobo transposable elements within the Drosophila melanogaster species complex: evidence from DNA sequencing. Mol Biol Evol 9:1050–1060
Sinkins SP, Godfray HC (2004) Use of Wolbachia to drive nuclear transgenes through insect populations. Proc Biol Sci 271:1421–1426
Sinkins SP, Gould F (2006) Gene drive systems for insect disease vectors. Nat Rev Genet 7:427–435
Sundararajan P, Atkinson PW, O’Brochta DA (1999) Transposable element interactions in insects: crossmobilization of hobo and Hermes. Insect Mol Biol 8:359–368
Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, Singh CM, Buchholz R, Demsky M, Fawcett R, Francis-Lang HL, Ryner L, Cheung LM, Chong A, Erickson C, Fisher WW, Greer K, Hartouni SR, Howie E, Jakkula L, Joo D, Killpack K, Laufer A, Mazzotta J, Smith RD, Stevens LM, Stuber C, Tan LR, Ventura R, Woo A, Zakrajsek I, Zhao L, Chen F, Swimmer C, Kopczynski C, Duyk G, Winberg ML, Margolis J (2004) A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet 36:283–287
Thomas DD, Donnelly CA, Wood RJ, Alphey LS (2000) Insect population control using a dominant, repressible, lethal genetic system. Science 287:2474–2476
Venken KJT, Bellen HJ (2005) Emerging technologies for gene manipulation in Drosophila melanogaster. Nat Rev Genet 6:167–178
Vreysen JB (2006) Prospects for area-wide integrated control of tsetse flies (Diptera:Glossinidae) and trypanosomosis in sub-Saharan Africa. Rev Soc Entomol Argent 65:1–21
Wilke ABB, Nimmo DD, St John O, Burini Kojin B, Capurro ML, Marrelli MT (2009) Mini-review: genetic enhancements to the sterile insect technique to control mosquito populations. AsPac J Mol Biol Biotechnol 17:65–74
Williams A, Harker N, Ktistaki E, Veiga-Fernandes H, Roderick K, Tolaini M, Norton T, Williams K, Kioussis D (2008) Position effect variegation and imprinting of transgenes in lymphocytes. Nucleic Acids Res 36:2320–2329
Windbichler N, Papathanos PA, Catteruccia F, Ranson H, Burt A, Crisanti A (2007) Homing endonuclease mediated gene targeting in Anopheles gambiae cells and embryos. Nucleic Acids Res 35:5922–5933
Wyss JH (2000) Screwworm eradication in the Americas. Ann NY Acad Sci 916:186–193
Yakob L, Kiss IZ, Bonsall MB (2008) A network approach to modeling population aggregation and genetic control of pest insects. Theor Popul Biol 74:324–331
Zwiebel LJ, Saccone G, Zacharopoulou A, Besansky NJ, Favia G, Collins FH, Louis C, Kafatos FC (1995) The white gene of Ceratitis capitata: a phenotypic marker for germline transformation. Science 270:2005–2008
Acknowledgments
The authors thank Gerald Franz and Jorge Hendrichs for support and advice and Ernst A. Wimmer for input and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scolari, F., Siciliano, P., Gabrieli, P. et al. Safe and fit genetically modified insects for pest control: from lab to field applications. Genetica 139, 41–52 (2011). https://doi.org/10.1007/s10709-010-9483-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-010-9483-7