Abstract
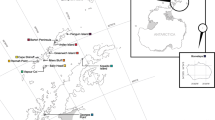
The north-central Patagonian coast is the sea lions most abundant area in Argentina. As occurs along the entire Atlantic coast, the distribution of breeding colonies at this smaller geographical scale is also patchy, showing at least three areas with breeding activity. We study the genetic structure and historical population dynamics of the species in five colonies in this area, analysing a 508 base-pair segment of the D-loop control region. Otaria flavescens showed 10 haplotypes with 12 polymorphic sites. The genealogical relationship between haplotypes revealed a shallow pattern of phylogeographic structure. The analysis of molecular variance showed significant differences between colonies, however, pairwise comparisons only indicate significant differences between a pair of colonies belonging to different breeding areas. The pattern of haplotype differentiation and the mismatch distribution analysis suggest a possible bottleneck that would have occurred 64,000 years ago, followed by a demographic expansion of the three southernmost colonies. Thus, the historical population dynamics of O. flavescens in north-central Patagonia appears to be closely related with the dynamics of the Late Pleistocene glaciations.



Similar content being viewed by others
References
Allnutt TR, Newton AC, Lara A, Premoli A, Armesto JJ, Vergara R, Gardner M (1999) Genetic variation in Fitzroya cupressoides (alerce), a threatened South American conifer. Mol Ecol 8:975–987
Allnutt TR, Newton AC, Premoli A, Lara A (2003) Genetic variation in the threatened South American conifer Pilgerodendron uviferum (Cupressaceae), detected using RAPD markers. Biol Conserv 114:245–253
Avise JC (2004) Molecular markers, natural history, and evolution. Sinauer Associates Inc, Sunderland
Báez AM, Scillato Yane GJ (1979) Late Cenozoic environmental changes in temperate Argentina. In: Duellman WE (ed) The South American Herpetofauna. Kansas University Press, Kansas, pp 141–156
Bandelt HJ, Forster P, Röhl A (1999) Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 16:37–48
Burg TM, Trites AW, Smith MJ (1999) Mitochondrial and microsatellite DNA analysis of harbour seal population structure in the northeast Pacific Ocean. Can J Zool 77:930–943
Campbell RA, Gales NJ, Lento GM, Baker CS (2008) Islands in the sea: extreme female natal site fidelity in the Australian sea lion, Neophoca cinerea. Biol Lett 4:139–142
Cappozzo HL, Campagna C, Monserrat J (1991) Sexual dimorphism in newborn southern sea lions. Mar Mamm Sci 7:385–394
Cornuet JM, Luikart G (1996) Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144:2001–2014
Dans SL, Crespo EA, Pedraza SN, Koen-Alonso M (2004) Recovery of the South American sea lion (Otaria flavescens) population in northern Patagonia. Can J Fish Aquat Sci 61:1681–1690
Dutech C, Joly HI, Jarne P (2004) Gene flow, historical population dynamics and genetic diversity within French Guianan populations of a rainforest tree species, Vouacapoua americana. Heredity 92:69–77
Excoffier L, Smouse P, Quattro J (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Excoffier L, Laval G, Schneider S (2005) Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online 1:47–50
Fernando P, Pfrender ME, Encalada SE, Lande R (2000) Mitochondrial DNA variation, phylogeography and population structure of the Asian elephant. Heredity 84:362–372
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Frenzel B (1973) Climatic fluctuations of the ice age. The Press of Case Western Reserve University, Cleveland & London
Harpending HC (1994) Signature of ancient population growth in a low-resolution mitochondrial DNA mismatch distribution. Hum Biol 66:591–600
Hasegawa M, Kishino H, Yano T (1985) Dating the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol 22:160–174
Herrnstadt C, Elson JL, Fahy E, Preston G, Turnbull DM, Anderson C, Ghosh SS, Olefsky JM, Beal MF, Davis DR, Howell N (2002) Reduced-median-network analysis of complete mitochondrial dna coding-region sequences for the major African, Asian, and European Haplogroups. Am J Hum Genet 70:1152–1171
Hewitt GM (1996) Some genetic consequences of ice ages and their role in divergence and speciation. Biol J Linn Soc 58:247–276
Hewitt GM (2004) Genetic consequences of climatic oscillations in the Quaternary. Philos Trans R Soc Lond B 359:183–195
Hoelzel AR, Halley J, O’Brien J, Campagna C, Arnbom T, Le Boeuf B, Ralls K, Dover GA (1993) Elephant seals genetic variation and the use of simulation models to investigate historical population bottlenecks. J Hered 84:443–449
Hoelzel AR, Campagna C, Arnbom T (2001) Genetic and morphometric differentiation between island and mainland southern elephant seal populations. Proc R Soc Lond B 268:325–332
Hoffman JI, Matson CW, Amos W, Loughlin TR, Bickham JW (2006) Deep genetic subdivision within a continuously distributed and highly vagile marine mammal, the Steller’s sea lion (Eumetopias jubatus). Mol Ecol 15:2821–2832
Howell N, Kubacka I, Mackay DA (1996) How rapidly does the human mitochondrial genome evolve? Am J Hum Genet 59:501–509
Kim I, Phillips CJ, Monjeau JA, Birney EC, Noack K, Pumo DE, Sikes RS, Dole JA (2002) Habitat islands, genetic diversity, and gene flow in a Patagonian rodent. Mol Ecol 7:667–678
Lamont MM, Vida JT, Harvey JT, Jeffries S, Brown R, Huber HH, DeLong R, Thomas WK (1996) Genetic substructure of the pacific harbor seal (Phoca vitulina richardsi) off Washington, Oregon, and California. Mar Mamm Sci 12:402–413
Latta RG, Mitton JB (1997) A comparison of population differentiation across four classes of gene marker in limber pine (Pinus flexilis James). Genetics 146:1153–1163
Lopez J, Yuki N, Masuda R, Modi W, O’brien SJ (1994) Numt, a recent transfer and tandem amplification of mitochondrial DNA to the nuclear genome of the domestic cat. J Mol Evol 39:174–190
Manly BFJ (1986) Multivariate statistical methods: a primer. Chapman & Hall, London
Matthee CA, Fourie F, Oosthuizen WH, Meyër MA, Tolley KA (2006) Mitochondrial DNA sequence data of the Cape fur seal (Arctocephalus pusillus pusillus) suggest that population numbers may be affected by climatic shifts. Mar Biol 148:899–905
Matthiopoulos J, Harwood J, Thomas L (2005) Metapopulation consequences of site fidelity for colonially breeding mammals and birds. J Anim Ecol 74:716–727
Mercer JH (1976) Glacial history of southernmost South America. Quat Res 6:125–166
Merilä J, Bjorklund M, Baker A (1997) Historical demography and present day population structure of the greenfinch, Carduelis chloris—an analysis of mtDNA control region sequences. Evolution 51:946–956
Milá B, Girman DJ, Kimura M, Smith TB (2000) Genetic evidence for the effect of a postglacial population expansion on the phylogeography of a North American songbird. Proc R Soc Lond B 267:1033–1040
Mizuno AW, Onuma M, Takahashi M, Ohtaishi N (2003) Population genetic structure of the spotted seal Phoca largha along the coast of Hokkaido, based on mitochondrial DNA sequences. Zool Sci 20:783–788
Muellner AN, Tremetsberger K, Stuessy T, Baeza CM (2005) Pleistocene refugia and recolonization routes in the southern Andes: insights from Hypochaeris palustris (Asteraceae, Lactuceae). Mol Ecol 14:203–212
Nei M, Maruyama T, Chakraborty R (1975) The bottleneck effect and genetic variability in populations. Evolution 29:1–10
Nielsen R, Wakeley J (2001) Distinguishing migration from isolation. Genetics 158:885–896
Phillips CD, Trujillo RG, Gelatt TS, Smolen MJ, Matson CW, Honeycutt RL, Patton JC, Bickham JW (2009) Assessing substitution patterns, rates and homoplasy at HVRI of Steller sea lions, Eumetopias jubatus. Mol Ecol 18:3379–3393
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818
Premoli AC, Souto CP, Rovere AE, Allnutt TR, Newton AC (2002) Patterns of isozyme variation as indicators of biogeographic history in Pilgerodendron uviferum (D. Don) Florín. Div Distr 8:57–66
Rasmussen PC (1987) Molts of the Rock Shag and new interpretations of the plumage sequence. Condor 89:760–766
Rasmussen PC (1991) Relationships between coastal South American King and Blue-eyed Shags. Condor 93:825–839
Reyes LM, Crespo EA, Szapkievich V (1999) Distribution and population size of the southern sea lion (Otaria flavescens) in central and southern Chubut, Patagonia, Argentina. Mar Mamm Sci 15:478–493
Rice WW (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rising JD, Avise JC (1993) An application of genealogical concordance principles to the taxonomy and evolutionary history of the sharp-tailed sparrow (Ammodramus caudacutus). Auk 110:844–856
Rogers AR (1995) Genetic evidence for a Pleistocene population expansion. Evolution 494:608–615
Rogers AR, Harpending H (1992) Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol 9:552–569
Ruzzante DE, Walde SJ, Cussac VE, Dalebout ML, Seibert J, Ortubay S, Habit E (2006) Phylogeography of the Percichthyidae (Pisces) in Patagonia: roles of orogeny, glaciation, and volcanism. Mol Ecol 15:2949–2968
Schiavini A, Crespo EA, Szapkievich V (2004) Status of the population of South American sea lion (Otaria flavescens Shaw, 1800) in southern Argentina. Mamm Biol 69:108–118
Siegel-Causey D (1997) Molecular variation and biogeography of rock shags. Condor 99:139–150
Slatkin M (1993) Isolation by distance in equilibrium and nonequilibrium populations. Evolution 47:264–269
Slatkin M, Hudson RR (1991) Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics 129:555–562
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial-DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Torroni A, Huoponen K, Francalacci P, Petrozzi M, Morelli L, Scozzari R, Obinu D, Savontaus ML, Wallace DC (1996) Classification of European mtDNAs from an analysis of three European populations. Genetics 144:1835–1850
Triant DA, DeWoody JA (2007) The occurrence, detection, and avoidance of mitochondrial DNA translocations in mammalian systematics and phylogeography. J Mam 88:908–920
Trujillo RG, Loughlin TR, Gemmell NJ, Patton JC, Bickham JW (2004) Variation in microsatellites and mtDNA across the range of the Steller sea lion, Eumetopias jubatus. J Mamm 85:338–346
Túnez JI, Centrón D, Cappozzo HL, Cassini MH (2007) Geographic distribution and diversity of mitochondrial DNA haplotypes in South American sea lions (Otaria flavescens) and fur seals (Arctocephalus australis). Mamm Biol 72:193–203
Túnez JI, Cappozzo HL, Cassini MH (2008) Natural and anthropogenic factors associated with the distribution of South American sea lion along the Atlantic coast. Hydrobiol 598:191–202
Vaz-Ferreira R (1982) Otaria flavescens (Shaw), South American sea lion. In: FAO and UNEP (eds) Mammals in the seas. Vol 4: Small cetaceans, seals, sirenians and otters. FAO Fisheries Series 5, Rome, pp 477–495
Vuilleumier BS (1971) Pleistocene changes in the fauna and flora of South America. Science 173:771–780
Vuilleumier F (1985) The forest birds of Patagonia: ecology, geography, speciation, endemism, and faunal history. Ornit Monogr 36:255–304
Wakeley J (1993) Substitution rate variation among sites in hypervariable region-1 of human mitochondrial-DNA. J Mol Evol 37:613–623
Wallace DC, Brown MD, Lott MT (1999) Mitochondrial DNA variation in human evolution and disease. Gene 238:211–230
Weber DS, Stewart BS, Lehman N (2004) Genetic consequences of a severe population bottleneck in the Guadalupe fur seal (Arctocephalus townsendi). J Hered 95:144–153
Wright S (1931) Evolution in Mendelian populations. Genetics 16:97–159
Wynen LP, Goldsworthy SD, Guinet C, Bester MN, Boyd IL, Gjertz I, Hofmeyr GJG, White RWG, Slade R (2000) Postsealing genetic variation and population structure of two species of fur seal (Arctocephalus gazelle and A. tropicalis). Mol Ecol 9:299–314
Zattara EE, Premoli AC (2005) Genetic structuring in Andean landlocked populations of Galaxias maculatus: effects of biogeographic history. J Biogeogr 32:5–14
Zink RM (1997) Phylogeographic studies of North American birds. In: Mindell DP (ed) Avian Molecular Evolution and Systematics. Academic Press, San Diego, pp 301–324
Acknowledgments
We thank José Saravia, Enrique Crespo, Fabián Pérez, Jorge O. Owen, Hugo Gómez, Rudy Bernabeu, Flavio Quintana, Rodolfo Werner and Valeria Szapkievich who assisted us during the sample collection. We also specially thanks to Prof. Rasmus Nielsen, who helped us in the MDIV analysis, and the three anonymous reviewers for their useful comments. This work was supported by the National Council of Scientific and Technical Research (CONICET) (PIP-01556 and PIP-05489), the Department of Basic Sciences from the University of Lujan, and PROFAUNA organization. JIT was supported by a PhD grant from CONICET. MHC is a researcher of the CONICET.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Túnez, J.I., Cappozzo, H.L., Nardelli, M. et al. Population genetic structure and historical population dynamics of the South American sea lion, Otaria flavescens, in north-central Patagonia. Genetica 138, 831–841 (2010). https://doi.org/10.1007/s10709-010-9466-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-010-9466-8