Abstract
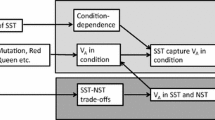
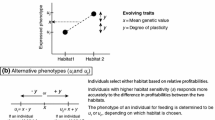
The lek paradox states that maintaining genetic variation necessary for ‘indirect benefit’ models of female choice is difficult, and two interrelated solutions have been proposed. ‘Genic capture’ assumes condition-dependence of sexual traits, while genotype-by-environment interactions (GEIs) offer an additional way to maintain diversity. However, condition-dependence, particularly with GEIs, implies that environmental variation can blur the relationship between male displays and offspring fitness. These issues have been treated separately in the past. Here we combine them in a population genetic model, and show that predictions change not only in magnitude but also in direction when the timing of dispersal between environments relative to the life cycle is changed. GEIs can dramatically improve the evolution of costly female preferences, but also hamper it if much dispersal occurs between the life history stage where condition is determined and mating. This situation also arises if selection or mutation rates are too high. In general, our results highlight that when evaluating any mechanism promoted as a potential resolution of the lek paradox, it is not sufficient to focus on its effects on genetic variation. It also has to be assessed to what extent the proposed mechanism blurs the association between male attractiveness and offspring fitness; the net balance of these two effects can be positive or negative, and often strongly context-dependent.


Similar content being viewed by others
References
Byers JA, Waits L (2006) Good genes sexual selection in nature. Proc Natl Acad Sci 103:16343–16345
Collins RD, Jang Y, Reinhold K, Greenfield MD (1999) Quantitative genetics of ultrasonic advertisement signalling in the lesser waxmoth Achroia grisella (Lepidoptera: Pyralidae). Heredity 83:644–651
Cotton S, Small J, Pomiankowski A (2006) Sexual selection and condition-dependent mate preferences. Curr Biol 16:R755–R765
Danielson-François AM, Kelly JK, Greenfield MD (2006) Genotype x environment interaction for male attractiveness in an acoustic moth: evidence for plasticity and canalization. J Evol Biol 19:532–542
David P, Björksten T, Fowler K, Pomiankowski A (2000) Condition-dependent signalling of genetic variation in stalk-eyes flies. Nature 406:186–188
Day T (2000) Sexual selection and the evolution of costly female preferences: spatial effects. Evolution 54:715–730
Ellner S, Hairston NG (1994) Role of overlapping generations in maintaining genetic variation in a fluctuating environment. Am Nat 143:403–417
Gillespie JH, Turelli M (1989) Genotype-environment interactions and the maintenance of polygenic variation. Genetics 10:253–280
Greenfield MD, Rodriguez RL (2004) Genotype-environment interaction and the reliability of mating signals. Anim Behav 68:1461–1468
Griffith SC, Owens IPF, Burke T (1999) Environmental determination of a sexually selected trait. Nature 400:358–360
Hine E, Chenoweth SF, Blows MW (2004) Multivariate quantitative genetics and the lek paradox: genetic variance in male sexually selected traits of Drosophila serrata under field conditions. Evolution 58:2754–2762
Hughes KA, Rodd FH, Reznick DN (2005) Genetic and environmental effects on secondary sex traits in guppies (Poecilia reticulata). J Evol Biol 18:35–45
Hunt J, Bussiere LF, Jennions MD, Brooks R (2004) What is genetic quality? Trends Ecol Evol 19:329–333
Jia FY, Greenfield MD, Collins RD (2000) Genetic variance of sexually selected traits in waxmoths: maintenance by genotype x environment interaction. Evolution 54:953–967
Kirkpatrick M, Ryan MJ (1991) The evolution of mating preferences and the paradox of the lek. Nature 350:33–38
Kokko H, Brooks R, McNamara JM, Houston AI (2002) The sexual selection continuum. Proc R Soc Lond B 269:1331–1340
Kokko H, Jennions MD, Brooks R (2006) Unifying and testing models of sexual selection. Ann Rev Ecol Evol Syst 37:43–66
Kokko H, Jennions MD, Houde A (2007) Evolution of frequency-dependent mate choice: keeping up with fashion trends. Proc R Soc Lond B 274:1317–1324
Lindström J (1999) Early development and fitness in birds and mammals. Trends Ecol Evol 14:343–348
Lindström J, Kokko H (2002) Cohort effects and population dynamics. Ecol Lett 5:338–344
McLain DK (2005) Female soldier beetles display a flexible preference for selectively favored male phenotypes. Evolution 59:1085–1095
Mills SC, Alatalo RV, Koskela E, Mappes J, Mappes T, Oksanen TA (2007) Signal reliability compromised by genotype by environment interaction is preserved by ontogenetic conflict. Evolution 61:1748–1757
Pomiankowski A, Iwasa Y, Nee S (1991) The evolution of costly mate preferences. 1. Fisher and biased mutation. Evolution 45:1422–1430
Proulx SR (2001) Female choice via indicator traits easily evolves in the face of recombination and migration. Evolution 55:2401–2411
Qvarnström A (1999) Genotype-by-environment interactions in the determination of the size of a secondary sexual character in the collared flycatcher (Ficedula albicollis). Evolution 53:1564–1572
Qvarnström A, Brommer JE, Gustafsson L (2006) Testing the genetics underlying the co-evolution of mate choice and ornament in the wild. Nature 441:84–86
Reinhold K (2004) Modeling a version of the good-genes hypothesis: female choice of locally adapted males. Org Divers Evol 4:157–163
Rodriguez RL, Greenfield MD (2003) Genetic variance and phenotypic plasticity in a component of female mate choice in an ultrasonic moth. Evolution 57:1304–1313
Rowe L, Houle D (1996) The lek paradox and the capture of genetic variance by condition dependent traits. Proc R Soc Lond B 263:1415–1421
Spichtig M, Kawecki TJ (2004) The maintenance (or not) of polygenic variation by soft selection in heterogeneous environments. Am Nat 164:70–84
Tomkins JL, Radwan J, Kotiaho JS, Tregenza T (2004) Genic capture and resolving the lek paradox. Trends Ecol Evol 19:323–328
Turelli M, Barton NH (2004) Polygenic variation maintained by balancing selection: Pleiotropy, sex-dependent allelic effects and GxE interactions. Genetics 166:1053–1079
Zhang X-S (2006) The phenotypic variance within plastic traits under migration-mutation-selection balance. Evolution 60:1125–1136
Acknowledgements
We thank the editors of this issue for inviting us to write this paper, two anonymous reviewers for their constructive suggestions, Michael Jennions for comments and coffee, Michael Greenfield and Jan Lindström for fruitful discussions, and the Academy of Finland for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kokko, H., Heubel, K. Condition-dependence, genotype-by-environment interactions and the lek paradox. Genetica 132, 209–216 (2008). https://doi.org/10.1007/s10709-007-9166-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-007-9166-1