Abstract
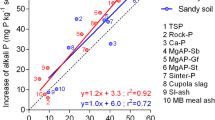
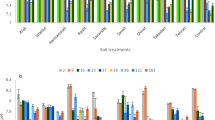
Phosphorus (P) nutrition of plants in croplands is managed by fertilization. Commercial P fertilizers are manufactured from phosphate rocks, which are non-renewable and the only fossil resource of P. As an alternative, P in human and animal wastes can be recovered and concentrated in products that can be used as P fertilizers. Here, we studied four recycled P products derived from pig manures (hereafter referred as “RPPM”) and another one derived from dairy effluents (“RPDE”). The RPDE product is composed of Ca–P (partly as hydroxyapatite, HA), while RPPM products include recovered struvite (ST) and Ca–P in variable proportions. The objective was to assess the ability of RPPM and RPDE products to increase available P in a range of soils differing in their characteristics (seven soils used), and to compare these recycled P products with a standard fertilizer [commercial triple super phosphates (TSP)], reference HA and reference ST. To this end, products were mixed to the soils and the mixtures were incubated at 75 % water holding capacity and 28 °C. After incubation, the amounts of phosphate ions (iP) in solution (QW) and isotopically exchangeable iP (E) in soils were quantified using an isotopic labeling (32P) and dilution procedure. In each soil, QW and E were significantly affected by treatments (control and P-treated soils) and increased due to the application of the different products. However, reference HA and RPDE products were generally less effective than TSP, reference ST and RPPM products. The soil response (variation in QW or E) in TSP treatment was compared to those in other treatments. It enabled the calculation of a relative effectiveness index. Relative effectiveness of HA and RPDE varied among soils (from 5 to 124 %) and increased with decreasing soil pH. Results however showed that the RPDE product tends to be more effective than reference HA, probably due to different degrees of crystallization of Ca–P. Relative effectiveness of RPPM products (80–116 %) was high in all soils and was similar to that of reference ST (90–104 %). To conclude, the present study suggests that RPDE products are effective only in acidic or slightly acidic soils. In contrast, P recycling from pig manures through chemical precipitation can provide effective P fertilizers, independently on soil conditions.




Similar content being viewed by others
References
Achat DL, Bakker MR, Augusto L, Saur E, Dousseron L, Morel C (2009) Evaluation of the phosphorus status of P-deficient podzols in temperate pine stands: combining isotopic dilution and extraction methods. Biogeochemistry 92:183–200
Achat DL, Augusto L, Morel C, Bakker MR (2011) Predicting available phosphate ions from physical–chemical soil properties in acidic sandy soils under pine forests. J Soils Sediments 11:452–466
Achat DL, Augusto L, Gallet-Budynek A, Bakker MR (2012) Drying-induced changes in phosphorus status of soils with contrasting soil organic matter contents—implications for laboratory approaches. Geoderma 187–188:41–48
AFNOR (1999) Qualité des sols, vol 1. Recueil de normes. (ed) AFNOR, Paris
Alvarez R, Evans LA, Milham PJ, Wilson MA (2004) Effects of humic material on the precipitation of calcium phosphate. Geoderma 118:245–260
Antonini S, Arias MA, Eichert T, Clemens J (2012) Greenhouse evaluation and environmental impact assessment of different urine-derived struvite fertilizers as phosphorus sources for plants. Chemosphere 89:1202–1210
Börling K, Barberis E, Otabbong E (2004) Impact of long-term inorganic phosphorus fertilization on accumulation, sorption and release of phosphorus in five Swedish soil profiles. Nutr Cycl Agroecosyst 69:11–21
Cabeza R, Steingrobe B, Römer W, Claassen N (2011) Effectiveness of recycled P products as P fertilizers, as evaluated in pot experiments. Nutr Cycl Agroecosyst 91:173–184
Capdevielle A, Sykorová E, Biscans B, Béline F, Daumer M-L (2013) Optimization of struvite precipitation in synthetic biologically treated swine wastewater—determination of the optimal process parameters. J Hazard Mater 244–245:357–369
Chien SH, Prochnow LI, Tu S, Snyder CS (2011) Agronomic and environmental aspects of phosphate fertilizers varying in source and solubility: an update review. Nutr Cycl Agroecosyst 89:229–255. doi:10.1007/s10705-010-9390-4
Cordell D, Drangert JO, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Chang 19:292–305
Daly K, Jeffrey D, Tunney H (2001) The effect of soil type on phosphorus sorption capacity and desorption dynamics in Irish grassland soils. Soil Use Manag 17:12–20
Daumer M-L, Santellani A-C, Capdevielle A, Diara A (2013) Phosphorus recycling as struvite from pig manure. Influence of process parameters. RAMIRAN, 15th international conference, Versailles, France, 3–5 June 2013
Demaria P, Sinaj S, Flisch R, Frossard E (2013) Soil properties and phosphorus isotopic exchangeability in cropped temperate soils. Commun Soil Sci Plant Anal 44:287–300
Devau N, Hinsinger P, Le Cadre E, Gérard F (2011) Root-induced processes controlling phosphate availability in soils with contrasted P-fertilized treatments. Plant Soil 348:203–218
Do Carmo Horta M, Torrent J (2007) Phosphorus desorption kinetics in relation to phosphorus forms and sorption properties of Portuguese acid soils. Soil Sci 172:631–638
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Fardeau JC (1993) Le phosphore assimilable des sols: sa représentation par un modèle fonctionnel à plusieurs compartiments. Agronomie 13:317–331
Fardeau JC (1996) Dynamics of phosphate in soils. An isotopic outlook. Fertil Res 45:91–100
Fardeau JC, Guiraud G, Marol C (1996) The role of isotopic techniques on the evaluation of the agronomic effectiveness of P fertilizers. Fertil Res 45:101–109
Frossard E, Sinaj S (1997) The isotope exchange kinetic technique: a method to describe the availability of inorganic nutrients, Applications to K, P, S and Zn. Isotopes Environ Health Stud 33:61–77
Frossard E, Fardeau JC, Brossard M, Morel JL (1994) Soil isotopically exchangeable phosphorus: a comparison between E and L values. Soil Sci Soc Am J 58:846–851
Gilbert N (2009) The disappearing nutrient. Nature 461:716–718
Gonzalez-Ponce R, Lopez-de-Sa EG, Plaza C (2009) Lettuce response to phosphorus fertilization with struvite recovered from municipal wastewater. HortScience 44:426–430
Gordon H, Haygarth PM, Bardgett RD (2008) Drying and rewetting effects on soil microbial community composition and nutrient leaching. Soil Biol Biochem 40:302–311
Guivarch A (2001) Valeur fertilisante à court terme du phosphore des boues de stations d’épuration urbaines, Ph. D. thesis, Institut National Polytechnique de Loraine, France, 306 pp
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Hutnik N, Piotrowski K, Wierzbowska B, Matynia A (2011) Continuous reaction crystallization of struvite from phosphate (V) solutions containing calcium ions. Cryst Res Technol 46:443–449
Johnston AE, Richards IR (2003) Effectiveness of different precipitated phosphates as phosphorus sources for plants. Soil Use Manag 19:44–49
Jungk A, Claassen N (1997) Ion diffusion in the soil-root system. Adv Agron 63:53–110
Kanabo IAK, Gilkes RJ (1987) The role of soil pH in the dissolution of phosphate rock fertilizers. Fertil Res 12:165–174
Kikuchi T, Okazaki M, Kimura SD, Motobayashi T, Baasansuren J, Hattori T, Abe T (2008) Suppressive effects of magnesium oxide materials on cadmium uptake and accumulation into rice grains II: suppression of cadmium uptake and accumulation into rice grains due to application of magnesium oxide materials. J Hazard Mater 154:294–299
Larsen S (1952) The use of 32P in studies on the uptake of phosphorus by plants. Plant Soil 4:1–10
Mañas A, Biscans B, Spérandio M (2011) Biologically induced phosphorus precipitation in aerobic granular sludge process. Water Res 45:3776–3786
Mañas A, Sperandio M, Decker F, Biscans B (2012) Location and chemical composition of microbially induced phosphorus precipitates in anaerobic and aerobic granular sludge. Environ Technol 33:2195–2209
Massey MS, Davis JG, Ippolito JA, Sheffield RE (2009) Effectiveness of recovered magnesium phosphates as fertilizers in neutral and slightly alkaline soils. Agron J 101:323–329
McKeague JA, Day JH (1966) Dithionite and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Can J Soil Sci 46:13–22
Mehra OP, Jackson ML (1960) Iron oxide removal from soils and clays by a dithionite–citrate system buffered with sodium bicarbonate. Clays Clay Miner 7:317–327
Messiga AJ, Ziadi N, Bélanger G, Morel C (2012) Process-based mass-balance modeling of soil phosphorus availability in a grassland fertilized with N and P. Nutr Cycl Agroecosyst 92:273–287
Morel C (2001) The effects of soil solution P and time on the transfer of P ions in soils from the IMPHOS European Network. In: Johnston AE, Ehlert PAI, Kueke M, Amar B, Jaggard KW, Morel C (eds) The effect of phosphate fertilizer management strategies on soil phosphorus status and crop yields in some European countries. Actes Editions, Rabat, pp 103–122
Morel C (2007) Mobilité et biodisponibilité du phosphore dans les sols cultivés: mécanismes, modélisation et diagnostic. Oceanis 33:51–74
Morel C, Fardeau JC (1990) Agronomical evaluation of phosphate fertilizer as a nutrient source of phosphorus for crops: isotopic procedure. Fertil Res 24:115–122
Morel C, Fardeau JC (1991) Phosphorus bioavailability of fertilizers: a predictive laboratory method for its evaluation. Fertil Res 28:1–9
Morel C, Plenchette C (1994) Is the isotopically exchangeable phosphate of a loamy soil the plant-available P? Plant Soil 158:287–297
Morel C, Tiessen H, Moir JO, Stewart JWB (1994) Phosphorus transformations and availability under cropping and fertilization assessed by isotopic exchange. Soil Sci Soc Am J 58:1439–1445
Morel C, Tunney H, Plenet D, Pellerin S (2000) Transfer of phosphate ions between soil and solution: perspectives in soil testing. J Environ Qual 29:20–59
Nanzer S, Oberson A, Berger L, Berset E, Hermann L, Frossard E (2014) The plant availability of phosphorus from thermo-chemically treated sewage sludge ashes as studied by 33P labeling techniques. Plant Soil 377:439–456
Oberson A, Tagmann HU, Langmeier M, Dubois D, Mäder P, Frossard E (2010) Fresh and residual phosphorus uptake by ryegrass from soils with different fertilization histories. Plant Soil 334:391–407
Rahman MdM, Liu YH, Kwag JH, Ra CS (2011) Recovery of struvite from animal wastewater and its nutrient leaching loss in soil. J Hazard Mater 186:2026–2030
Santellani A-C, Capdevielle A, Diara A, Daumer M-L (2013) Study of parameters influencing the process of phosphorus recovery in synthetic biologically treated pig effluent in pilot lab scale. RAMIRAN, 15th international conference, Versailles, France, 3–5 June 2013
Saunders WMH, Williams EG (1955) Observations on the determination of total organic phosphorus in soils. J Soil Sci 6:247–267
Six L, Smolders E, Merckx R (2012) The performance of DGT versus conventional soil phosphorus tests in tropical soils—maize and rice responses to P application. Plant Soil. doi:10.1007/s11104-012-1375-4
Strauss R, Brümmer GW, Barrow NJ (1997) Effects of crystallinity of goethite: II. Rates of sorption and desorption of phosphate. Eur J Soil Sci 48:101–114
Stroia C, Morel C, Jouany C (2011) Nitrogen fertilization effects on grassland soil acidification: consequences on diffusive phosphorus ions. Soil Sci Soc Am J 75:112–120
SYSTAT Software, Inc. (2000) Version 10. SYSTAT Software, Chicago, IL
Van Veldhoven PP, Mannaerts GP (1987) Inorganic and organic phosphate measurements in the nanomolar range. Anal Biochem 161:45–48
Warren GP, Robinson JS, Someus E (2009) Dissolution of phosphorus from animal bone char in 12 soils. Nutr Cycl Agroecosyst 84:167–178
Acknowledgments
The present study was performed in the frame of the Phosph’Or Project (ANR–ECOTECH; “Développement de procédés de recyclage du phosphore sous une forme valorisable en agriculture. Analyse de la valeur fertilisante phosphatée de produits de recyclage”). We thank the French National Research Agency (ANR) and INSA Toulouse for financial supports and INRA Bordeaux for laboratory facilities. Stéphane Thunot (UMR 1391 ISPA, INRA Bordeaux), Thierry Morvan (UMR 1069 SAS, INRA/Agrocampus Rennes), Daniel Hanocq and Yvon Lambert (Chambres d’Agriculture de Bretagne) are thanked for providing us soil samples. We also thank two reviewers and the editor for their useful comments and Mark Bakker for checking the English language.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Achat, D.L., Daumer, ML., Sperandio, M. et al. Solubility and mobility of phosphorus recycled from dairy effluents and pig manures in incubated soils with different characteristics. Nutr Cycl Agroecosyst 99, 1–15 (2014). https://doi.org/10.1007/s10705-014-9614-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-014-9614-0