Abstract
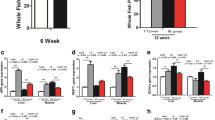
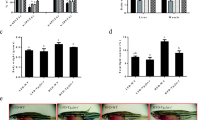
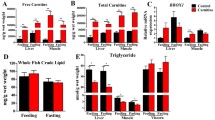
The adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL)–mediated lipolysis play important roles in lipid catabolism. ATGL is considered the central rate-limiting enzyme in the mobilization of fatty acids in mammals. Currently, severe fat accumulation has been commonly detected in farmed fish globally. However, the ATGL-mediated lipolysis and the potential synergy among ATGL, HSL, and autophagy, which is another way for lipid breakdown, have not been intensively understood in fish. In the present study, we added Atglistatin as an ATGL-specific inhibitor into the zebrafish diet and fed to the fish for 5 weeks. The results showed that the Atglistatin-treated fish exhibited severe fat deposition, reduced oxygen consumption, and fatty acid β-oxidation, accompanied with increased oxidative stress and inflammation. Furthermore, the Atglistatin-treated fish elevated total and phosphorylation protein expressions of HSL. However, the free fatty acids and lipase activities in organs were still systemically reduced in the Atglistatin-treated fish, and the autophagy marker LC3 was also decreased in the liver. On the other hand, glycogenolysis was stimulated but blood glucose was higher in the Atglistatin-treated fish. The transcriptomic analysis also provided the hint that the protein turnover efficiency in Atglistatin-treated fish was likely to be accelerated, but the protein content in whole fish was not affected. Taken together, ATGL plays crucial roles in energy homeostasis such that its inhibition causes loss of lipid-sourced energy production, which cannot be compensated by activation of HSL, autophagy, and utilization of other nutrients.







Similar content being viewed by others
Data availability
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ahmadian M, Duncan RE, Varady KA, Frasson D, Hellerstein MK, Birkenfeld AL, Samuel VT, Shulman GI, Wang Y, Kang C (2009) Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity. Diabetes 58:855–866. https://doi.org/10.2337/db08-1644
Attané C, Peyot ML, Lussier R, Poursharifi P, Zhao S, Zhang D, Morin J, Pineda M, Wang S, Dumortier O (2016) A beta cell ATGL-lipolysis/adipose tissue axis controls energy homeostasis and body weight via insulin secretion in mice. Diabetologia 59:2654–2663. https://doi.org/10.1007/s00125-016-4105-2
Cerk IK, Wechselberger L, Oberer M (2018) Adipose triglyceride lipase regulation: an overview. Curr Protein Pept Sci 19:221–233. https://doi.org/10.2174/1389203718666170918160110
Chen QL, Luo Z, Song YF, Wu K, Huang C, Pan YX, Zhu QL (2014) Hormone-sensitive lipase in yellow catfish Pelteobagrus fulvidraco: molecular characterization, mRNA tissue expression and transcriptional regulation by leptin in vivo and in vitro. Gen Comp Endocrinol 206:130–138. https://doi.org/10.1016/j.ygcen.2014.06.031
Dai YJ, Liu WB, Li XF, Zhou M, Xu C, Qian Y, Jiang GZ (2018) Molecular cloning of adipose triglyceride lipase (ATGL) gene from blunt snout bream and its expression after LPS-induced TNF-α factor. Fish Physiol Biochem 44:1143–1157. https://doi.org/10.1007/s10695-018-0502-4
Du ZY, Clouet P, Huang L, Degrace P, Zheng W, He J, Tian L, Liu Y (2008) Utilization of different dietary lipid sources at high level in herbivorous grass carp (Ctenopharyngodon idella): mechanism related to hepatic fatty acid oxidation. Aquac Nutr 14:77–92. https://doi.org/10.1111/j.1365-2095.2007.00507.x
Du ZY, Ma T, Liaset B, Keenan AH, Araujo P, Lock EJ, Demizieux L, Degrace P, Frøyland L, Kristiansen K, Madsen L (2013) Dietary eicosapentaenoic acid supplementation accentuates hepatic triglyceride accumulation in mice with impaired fatty acid oxidation capacity. Biochim Biophys Acta 1831:291–299. https://doi.org/10.1016/j.bbalip.2012.10.002
Faillaci F, Milosa F, Critelli RM, Turola E, Schepis F, Villa E (2018) Obese zebrafish: a small fish for a major human health condition. Anim Model Exp Med 1:255–265. https://doi.org/10.1002/ame2.12042
Fredrikson G, Tornqvist H, Belfrage P (1986) Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Biochim Biophys Acta 876:288–293. https://doi.org/10.1016/0005-2760(86)90286-9
Goeritzer M, Vujic N, Schlager S, Chandak PG, Korbelius M, Gottschalk B, Leopold C, Obrowsky S, Rainer S, Doddapattar P (2015) Active autophagy but not lipophagy in macrophages with defective lipolysis. Biochim Biophys Acta 1851:1304–1316. https://doi.org/10.1016/j.bbalip.2015.06.005
Guo F, Ma Y, Kadegowda AK, Betters JL, Xie P, Liu G, Liu X, Miao H, Ou J, Su X (2013) Deficiency of liver comparative gene identification-58 causes steatohepatitis and fibrosis in mice. J Lipid Res 54:2109–2120. https://doi.org/10.1194/jlr.M035519
Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, Sattler W, Magin TM, Wagner EF, Zechner R (2002) Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem 277:4806–4815. https://doi.org/10.1074/jbc.M110355200
Haemmerle G, Lass A, Zimmermann R, Gorkiewicz G, Meyer C, Rozman J, Heldmaier G, Maier R, Theussl C, Eder S (2006) Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312:734–737. https://doi.org/10.1126/science.1123965
Haemmerle G, Moustafa T, Woelkart G, Büttner S, Schmidt A, Van De Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K (2011) ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat Med 17:1076–1085. https://doi.org/10.1038/nm.2439
Han SL, Wang J, Li LY, Lu DL, Chen LQ, Zhang ML, Du ZY (2020) The regulation of rapamycin on nutrient metabolism in Nile tilapia fed with high-energy diet. Aquaculture 520:734975. https://doi.org/10.1016/j.aquaculture.2020.734975
Kajimura J, Ito R, Manley NR, Hale LP (2016) Optimization of single-and dual-color immunofluorescence protocols for formalin-fixed, paraffin-embedded archival tissues. J Histochem Cytochem 64:112–124. https://doi.org/10.1369/0022155415610792
Kienesberger PC, Lee D, Pulinilkunnil T, Brenner DS, Cai L, Magnes C, Koefeler HC, Streith IE, Rechberger GN, Haemmerle G (2009) Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling. J Biol Chem 284:30218–30229. https://doi.org/10.1074/jbc.M109.047787
Kraemer FB, Shen WJ (2006) Hormone-sensitive lipase knockouts. Nutr Metab 3:12. https://doi.org/10.1186/1743-7075-3-12
Lafontan M, Langin D (2009) Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 48:275–297. https://doi.org/10.1016/j.plipres.2009.05.001
Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T (2008) A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A 105:3374–3379. https://doi.org/10.1073/pnas.0712145105
Lee JM, Wagner M, Xiao R, Kim KH, Feng D, Lazar MA, Moore DD (2014) Nutrient-sensing nuclear receptors coordinate autophagy. Nature 516:112–115. https://doi.org/10.1038/nature13961
Li JM, Li LY, Qin X, Ning LJ, Lu DL, Li DL, Zhang ML, Wang X, Du ZY (2017) Systemic regulation of L-carnitine in nutritional metabolism in zebrafish, Danio rerio. Sci Rep 7:40815. https://doi.org/10.1038/srep40815
Liu D, Mai K, Ai Q (2015) Tumor necrosis factor alpha is a potent regulator in fish adipose tissue. Aquaculture 436:65–71. https://doi.org/10.1016/j.aquaculture.2014.10.030
Martinez-Lopez N, Garcia-Macia M, Sahu S, Athonvarangkul D, Liebling E, Merlo P, Cecconi F, Schwartz GJ, Singh R (2016) Autophagy in the CNS and periphery coordinate lipophagy and lipolysis in the brown adipose tissue and liver. Cell Metab 23:113–127. https://doi.org/10.1016/j.cmet.2015.10.008
Mayer N, Schweiger M, Romauch M, Grabner GF, Eichmann TO, Fuchs E, Ivkovic J, Heier C, Mrak I, Lass A (2013) Development of small-molecule inhibitors targeting adipose triglyceride lipase. Nat Chem Biol 9:785–787. https://doi.org/10.1038/nchembio.1359
Ning LJ, He AY, Li JM, Lu DL, Jiao JG, Li LY, Li DL, Zhang ML, Chen LQ, Du ZY (2016) Mechanisms and metabolic regulation of PPARα activation in Nile tilapia (Oreochromis niloticus). Biochim Biophys Acta 1861:1036–1048. https://doi.org/10.1016/j.bbalip.2016.06.005
Obrowsky S, Chandak PG, Patankar JV, Povoden S, Schlager S, Kershaw EE, Bogner-Strauss JG, Hoefler G, Levak-Frank S, Kratky D (2013) Adipose triglyceride lipase is a TG hydrolase of the small intestine and regulates intestinal PPARα signaling. J Lipid Res 54:425–435. https://doi.org/10.1194/jlr.M031716
Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG (2011) Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology 53:116–126. https://doi.org/10.1002/hep.24006
Parhofer KG (2015) Interaction between glucose and lipid metabolism: more than diabetic dyslipidemia. Diabetes Metab J 39:353–362. https://doi.org/10.4093/dmj.2015.39.5.353
Perry RJ, Samuel VT, Petersen KF, Shulman GI (2014) The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510:84–91. https://doi.org/10.1038/nature13478
Peyot ML, Guay C, Latour MG, Lamontagne J, Lussier R, Pineda M, Ruderman NB, Haemmerle G, Zechner R, Joly É (2009) Adipose triglyceride lipase is implicated in fuel-and non-fuel-stimulated insulin secretion. J Biol Chem 284:16848–16859. https://doi.org/10.1074/jbc.M109.006650
Raben DM, Baldassare JJ (2005) A new lipase in regulating lipid mobilization: hormone-sensitive lipase is not alone. Trends Endocrinol Metab 16:35–36. https://doi.org/10.1016/j.tem.2005.01.009
Rambold AS, Cohen S, Lippincott-Schwartz J (2015) Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell 32:678–692. https://doi.org/10.1016/j.devcel.2015.01.029
Reid BN, Ables GP, Otlivanchik OA, Schoiswohl G, Zechner R, Blaner WS, Goldberg IJ, Schwabe RF, Chua SC, Huang LS (2008) Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem 283:13087–13099. https://doi.org/10.1074/jbc.M800533200
Reue K (2011) A thematic review series: lipid droplet storage and metabolism: from yeast to man. J Lipid Res 52:1865–1868. https://doi.org/10.1194/jlr.E020602
Rodriguez JA, Ali YB, Abdelkafi S, Mendoza LD, Leclaire J, Fotiadu F, Buono G, Carrière F, Abousalham A (2010) In vitro stereoselective hydrolysis of diacylglycerols by hormone-sensitive lipase. Biochim Biophys Acta 1801:77–83. https://doi.org/10.1016/j.bbalip.2009.09.020
Sathyanarayan A, Mashek MT, Mashek DG (2017) ATGL promotes autophagy/lipophagy via SIRT1 to control hepatic lipid droplet catabolism. Cell Rep 19:1–9. https://doi.org/10.1016/j.celrep.2017.03.026
Schoiswohl G, Stefanovic-Racic M, Menke MN, Wills RC, Surlow BA, Basantani MK, Sitnick MT, Cai L, Yazbeck CF, Stolz DB (2015) Impact of reduced ATGL-mediated adipocyte lipolysis on obesity-associated insulin resistance and inflammation in male mice. Endocrinology 156:3610–3624. https://doi.org/10.1210/en.2015-1322
Schreiber R, Xie H, Schweiger M (2019) Of mice and men: the physiological role of adipose triglyceride lipase (ATGL). Biochim Biophys Acta Mol Cell Biol Lipids 1864:880–899. https://doi.org/10.1016/j.bbalip.2018.10.008
Schulze RJ, Sathyanarayan A, Mashek DG (2017) Breaking fat: the regulation and mechanisms of lipophagy. Biochim Biophys Acta 1862:1178–1187. https://doi.org/10.1016/j.bbalip.2017.06.008
Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R (2006) Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 281:40236–40241. https://doi.org/10.1074/jbc.M608048200
Schweiger M, Romauch M, Schreiber R, Grabner GF, Hütter S, Kotzbeck P, Benedikt P, Eichmann TO, Yamada S, Knittelfelder O (2017) Pharmacological inhibition of adipose triglyceride lipase corrects high-fat diet-induced insulin resistance and hepatosteatosis in mice. Nat Commun 8:14859. https://doi.org/10.1038/ncomms14859
Singh R, Cuervo AM (2012) Lipophagy: connecting autophagy and lipid metabolism. Int J Cell Biol 2012:282041–282012. https://doi.org/10.1155/2012/282041
Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL (2006) ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep 7:106–113. https://doi.org/10.1038/sj.embor.7400559
Stone DA (2003) Dietary carbohydrate utilization by fish. Rev Fish Sci 11:337–369. https://doi.org/10.1080/10641260390260884
Sun J, Ji H, Li XX, Shi XC, Du ZY, Chen LQ (2016) Lipolytic enzymes involving lipolysis in teleost: Synteny, structure, tissue distribution, and expression in grass carp (Ctenopharyngodon idella). Comp Biochem Physiol B Biochem Mol Biol 198:110–118. https://doi.org/10.1016/j.cbpb.2016.04.008
Turpin SM, Hoy AJ, Brown RD, Rudaz CG, Honeyman J, Matzaris M, Watt MJ (2011) Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia 54:146–156. https://doi.org/10.1007/s00125-010-1895-5
Van Herpen NA, Schrauwen-Hinderling VB (2008) Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav 94:231–241. https://doi.org/10.1016/j.physbeh.2007.11.049
Wang X, Wang Y, Li Y (2013) Adipose triglyceride lipase (ATGL) clone, expression pattern, and regulation by different lipid sources and lipid levels in large yellow croaker (Pseudosciaena crocea R.). Mar Biotechnol 15:197–205. https://doi.org/10.1007/s10126-012-9477-9
Wang J, Han SL, Li LY, Lu DL, Limbu SM, Li DL, Zhang ML, Du ZY (2018) Lipophagy is essential for lipid metabolism in fish. Sci Bull 63:879–882. https://doi.org/10.1016/j.scib.2018.05.026
Wang J, Han SL, Lu DL, Li LY, Limbu SM, Li DL, Zhang ML, Du ZY (2019) Inhibited lipophagy suppresses lipid metabolism in zebrafish liver cells. Front Physiol 10:1077. https://doi.org/10.3389/fphys.2019.01077
Williams MB, Watts SA (2019) Current basis and future directions of zebrafish nutrigenomics. Genes Nutr 14:34. https://doi.org/10.1186/s12263-019-0658-2
Wu JW, Wang SP, Alvarez F, Casavant S, Gauthier N, Abed L, Soni KG, Yang G, Mitchell GA (2011) Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology 54:122–132. https://doi.org/10.1002/hep.24338
Wu JW, Wang SP, Casavant S, Moreau A, Yang GS, Mitchell GA (2012) Fasting energy homeostasis in mice with adipose deficiency of desnutrin/adipose triglyceride lipase. Endocrinology 153:2198–2207. https://doi.org/10.1210/en.2011-1518
Xia B, Cai GH, Yang H, Wang SP, Mitchell GA, Wu JW (2017) Adipose tissue deficiency of hormone-sensitive lipase causes fatty liver in mice. PLoS Genet 13:e1007110. https://doi.org/10.1371/journal.pgen.1007110
Xie P, Guo F, Ma Y, Zhu H, Wang F, Xue B, Shi H, Yang J, Yu L (2014) Intestinal Cgi-58 deficiency reduces postprandial lipid absorption. PLoS One 9:e91652. https://doi.org/10.1371/journal.pone.0091652
Yan J, Liao K, Wang T, Mai K, Xu W, Ai Q (2015) Dietary lipid levels influence lipid deposition in the liver of large yellow croaker (Larimichthys crocea) by regulating lipoprotein receptors, fatty acid uptake and triacylglycerol synthesis and catabolism at the transcriptional level. PLoS One 10:e0129937. https://doi.org/10.1371/journal.pone.0129937
Yang L, Li P, Fu S, Calay ES, Hotamisligil GS (2010) Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab 11:467–478. https://doi.org/10.1016/j.cmet.2010.04.005
Yang BY, Zhai G, Gong YL, Su JZ, Peng XY, Shang GH, Han D, Jin JY, Liu HK, Du ZY, Yin Z, Xie SQ (2017) Different physiological roles of insulin receptors in mediating nutrient metabolism in zebrafish. Am J Physiol Endocrinol Metab 315:E38–E51. https://doi.org/10.1152/ajpendo.00227.2017
Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F (2012) FAT SIGNALS-lipases and lipolysis in lipid metabolism and signaling. Cell Metab 15:279–291. https://doi.org/10.1016/j.cmet.2011.12.018
Zechner R, Madeo F, Kratky D (2017) Cytosolic lipolysis and lipophagy: two sides of the same coin. Nat Rev Mol Cell Biol 18:671–684. https://doi.org/10.1038/nrm.2017.76
Zhang Z, Yao Z, Chen Y, Qian L, Jiang S, Zhou J, Shao J, Chen A, Zhang F, Zheng S (2018) Lipophagy and liver disease: new perspectives to better understanding and therapy. Biomed Pharmacother 97:339–348. https://doi.org/10.1016/j.biopha.2017.07.168
Zhang X, Zhang CC, Yang H, Soni KG, Wang SP, Mitchell GA, Wu JW (2019) An epistatic interaction between Pnpla2 and Lipe reveals new pathways of adipose tissue lipolysis. Cells 8:395. https://doi.org/10.3390/cells8050395
Zhao T, Wu K, Hogstrand C, Xu YH, Chen GH, Wei CC, Luo Z (2019) Lipophagy mediated carbohydrate-induced changes of lipid metabolism via oxidative stress, endoplasmic reticulum (ER) stress and ChREBP/PPARγ pathways. Cell Mol Life Sci 77:1987–2003. https://doi.org/10.1007/s00018-019-03263-6
Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306:1383–1386. https://doi.org/10.1126/science.1100747
Funding
This work was financed by the National Natural Science Fund of China (31830102) and the Program of Shanghai Academic Research Leader (19XD1421200).
Author information
Authors and Affiliations
Contributions
ZY. D. and SL. H. conceived the study and designed the experiments. SL. H. carried out the experiments, analyzed the data, and wrote the manuscript. Y. L., LQ. C., and ML. Z. contributed reagents/materials/analysis tools. SL. H., S. M. L., and ZY. D. wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
All experiments were conducted strictly under the Guidance of the Care and Use of Laboratory Animals in China. This study was approved by the Committee on the Ethics of Animal Experiments of East China Normal University (Approval ID: F20140101).
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 254 kb)
Rights and permissions
About this article
Cite this article
Han, SL., Liu, Y., Limbu, S.M. et al. The reduction of lipid-sourced energy production caused by ATGL inhibition cannot be compensated by activation of HSL, autophagy, and utilization of other nutrients in fish. Fish Physiol Biochem 47, 173–188 (2021). https://doi.org/10.1007/s10695-020-00904-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-020-00904-7