Abstract
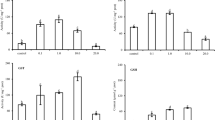
The effects of ammonia exposure and recovery on oxidative stress parameters and histology of juvenile Brazilian flounder Paralichthys orbignyanus were evaluated. The fish were exposed to 0.12, 0.28 and 0.57 mg NH3-N L−1, plus a control, for 10 days followed by the same recovery time in ammonia-free water. Gill, liver and muscle samples (n = 9) were collected after 1, 5 and 10 days of exposure and after recovery for oxidative stress analysis (antioxidant capacity against peroxyl radicals (ACAP); glutathione S-transferase (GST) activity; lipoperoxidation levels measured through thiobarbituric acid reactive substances (TBARS) content). For histological assessment, gill, liver and brain samples were collected. Exposure to all NH3-N concentrations induced different time- and dose-dependent changes in oxidative stress parameters. Reduced antioxidant capacity of the liver and muscle and enhanced TBARS levels in the gills and liver were demonstrated. Differently, a high ammonia concentration elicited lower hepatic TBARS levels. Enhanced GST activity in all organs and increased antioxidant capacity of the gills were also observed. No ammonia-induced histopathological effects were demonstrated. After recovery, most parameters (liver ACAP, GST activity in the muscle and liver and TBARS in the gills) returned to baseline levels. However, liver TBARS and gill GST activity remained altered 0.57 mg NH3-N L−1 treatment. The recovery period also led to a decrease in gill antioxidant capacity and an increase in muscle antioxidant capacity. In conclusion, a concentration of 0.12 mg NH3-N L−1 induces oxidative stress and antioxidant responses in juvenile Brazilian flounder. Moreover, a 10-day recovery period is not sufficient to restore fish homeostasis.



Similar content being viewed by others
References
Ackerman PA, Wicks BJ, Iwama GK, Randall DJ (2006) Low levels of environmental ammonia increase susceptibility to disease in chinook salmon smolts. Physiol Biochem Zool 79:695–707. doi:10.1086/504615
Amado LL, Garcia ML, Ramos PB, Freitas RF, Zafalon B, Ferreira JL, Yunes JS, Monserrat JM (2009) A method to measure total antioxidant capacity against peroxyl radicals in aquatic organisms: application to evaluate microcystins toxicity. Sci Total Environ 407:2115–2123. doi:10.1016/j.scitotenv.2008.11.038
Baldisserotto B, Martos-Sitcha JA, Menezes CC, Toni C, Prati RL, Garcia LO, Salbego J, Mancera JM, Martínez-Rodríguez G (2014) The effects of ammonia and water hardness on the hormonal, osmoregulatory and metabolic responses of the freshwater silver catfish Rhamdia quelen. Aquat Toxicol 152:341–352. doi:10.1016/j.aquatox.2014.04.023
Bendschneider K, Robinson RJ (1952) A new spectrophotometric method for the determination of nitrite in sea water. J Mar Res 11:87–96
Bianchini A, Wasielesky W, Filho KC (1996) Toxicity of nitrogenous compounds to juveniles of flatfish Paralichthys orbignyanus. Bull Environ Contam Toxicol 56:453–459. doi:10.1007/s001289900065
Blanchette B, Feng X, Singh BR (2007) Marine glutathione S-transferases. Mar Biotechnol 9:513–542. doi:10.1007/s10126-007-9034-0
Bolasina SN (2011) Stress response of juvenile flounder (Paralichthys orbignyanus, Valenciennes 1839), to acute and chronic stressors. Aquaculture 313:140–143. doi:10.1016/j.aquaculture.2011.01.011
Carvalho-Neta RN, Abreu-Silva AL (2013) Glutathione S-transferase as biomarker in Sciades herzbergii (Siluriformes: Ariidae) for environmental monitoring: the case study of São Marcos Bay, Maranhão, Brazil. Lat Am J Aquat Res 41:217–225. doi:10.3856/vol41-issue2-fulltext-2
Cheng CH, Yang FF, Ling RZ, Liao SA, Miao YT, Ye CX, Wang AL (2015) Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat Toxicol 164:61–71. doi:10.1016/j.aquatox.2015.04.004
Ching B, Chew SF, Wong WP, Ip YK (2009) Environmental ammonia exposure induces oxidative stress in gills and brain of Boleophthalmus boddarti (mudskipper). Aquat Toxicol 95:203–212. doi:10.1016/j.aquatox.2009.09.004
Colt J (2002) List of spreadsheets prepared as a complement. In: Wedemeyer GA (ed) Fish hatchery management, 2nd edn. American Fish Society Publication http://www.fisheries.org/afs/hatchery.html. Accessed 10 April 2013
Da Rocha AM, de Freitas DP, Burns M, Vieira JP, de la Torre FR, Monserrat JM (2009) Seasonal and organ variations in antioxidant capacity, detoxifying competence and oxidative damage in freshwater and estuarine fishes from Southern Brazil. Comp Biochem Phys C Toxicol Pharmacol 150:512–520. doi:10.1016/j.cbpc.2009.07.012
Dong X, Zhang X, Qin J, Zong S (2013) Acute ammonia toxicity and gill morphological changes of Japanese flounder Paralichthys olivaceus in normal versus supersaturated oxygen. Aquac Res 44:1752–1759. doi:10.1111/j.1365-2109.2012.03181.x
Eaton AD, Clesceri LS, Rice EW, Greenberg AE (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington, D.C.
Enamorado AD, Martins AC, Flores JA, Tesser MB, Caldas SS, Primel EG, Monserrat JM (2015) Biochemical responses over time in common carp Cyprinus carpio (Teleostei, Cyprinidae) during fed supplementation with α-lipoic acid. Comp Biochem Phys A Mol Integr Physiol 188:9–16. doi:10.1016/j.cbpa.2015.05.023
FAO–Food and Agriculture Organization of the United Nations (2016) The state of world fisheries and aquaculture—contributing to food security and nutrition for all, Rome http://www.fao.org/3/a-i5555e.pdf. Accessed 13 February 2017
Farag AM, May T, Marty GD, Easton M, Harper DD, Little EE, Cleveland L (2006) The effect of chronic chromium exposure on the health of chinook salmon (Oncorhynchus tshawytscha). Aquat Toxicol 76:246–257. doi:10.1016/j.aquatox.2005.09.011
Farzad R, Inanlou DN, Cohan RA, Ghorbani M (2015) Effects of the industrial pollution on glutathione S-tranferase in the liver of rainbow trout. Bulg Chem Commun 47:720–724
Figueiredo JL, Menezes NA (2000) Manual de peixes marinhos do sudeste do Brasil. São Paulo, Museu de Zoologia/USP
Frances J, Nowak BF, Allan GL (2000) Effects of ammonia on juvenile silver perch (Bidyanus bidyanus). Aquaculture 183:95–103. doi:10.1016/S0044-8486(99)00286-0
Fridovich I (2004) Mitochondria: are they the seat of senescence? Aging Cell 3:13–16. doi:10.1046/j.1474-9728.2003.00075.x
Garcia LO, Okamoto MH, Riffel AP, Saccol EM, Pavanato MA, Sampaio LA (2015) Oxidative stress parameters in juvenile Brazilian flounder Paralichthys orbignyanus (Valenciennes, 1839) (Pleuronectiformes: Paralichthyidae) exposed to cold and heat shocks. Neotrop Ichthyol 13:607–612. doi:10.1590/1982-0224-20140148
Gottschalk S, Zwingmann C (2009) Altered fatty acid metabolism and composition in cultured astrocytes under hyperammonemic conditions. J Neurochem 109:258–264. doi:10.1111/j.1471-4159.2009.05985.x
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione S-transferases. Methods Enzymol 77:398–405
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Halliwell B, Gutteridge JM (2015) Free radicals in biology and medicine. Oxford University Press, USA
Hegazi MM, Attia ZI, Ashour OA (2010) Oxidative stress and antioxidant enzymes in liver and white muscle of Nile tilapia juveniles in chronic ammonia exposure. Aquat Toxicol 99:118–125. doi:10.1016/j.aquatox.2010.04.007
Hermes-Lima M, Moreira DC, Rivera-Ingraham GA, Giraud-Billoud M, Genaro-Mattos TC, Campos ÉG (2015) Preparation for oxidative stress under hypoxia and metabolic depression: revisiting the proposal two decades later. Free Radical Bio Med 89:1122–1143. doi:10.1016/j.freeradbiomed.2015.07.156
Intergovernmental Oceanographic Commission (1983) Chemical methods for use in marine environmental monitoring. UNESCO, Paris
Ip YK, Chew SF (2010) Ammonia production, excretion, toxicity, and defense in fish: a review. Front Physiol 1:134. doi:10.3389/fphys.2010.00134
Kim SH, Kim JH, Park MA, Hwang SD, Kang JC (2015) The toxic effects of ammonia exposure on antioxidant and immune responses in rockfish, Sebastes schlegelii during thermal stress. Environ Toxicol Pharmacol 40:954–959. doi:10.1016/j.etap.2015.10.006
Klinger D, Naylor R (2012) Searching for solutions in aquaculture: charting a sustainable course. Annu Rev Environ Resour 37:247–276. doi:10.1146/annurev-environ-021111-161531
Kolarevic J, Takle H, Felip O, Ytteborg E, Selset R, Good CM, Baeverfjord G, Asgård T, Terjesen BF (2012) Molecular and physiological responses to long-term sub-lethal ammonia exposure in Atlantic salmon (Salmo salar). Aquat Toxicol 124:48–57. doi:10.1016/j.aquatox.2012.07.003
Kütter MT, Romano LA, Ventura-Lima J, Tesser MB, Monserrat JM (2014) Antioxidant and toxicological effects elicited by alpha-lipoic acid in aquatic organisms. Comp Biochem Phys C Toxicol Pharmacol 162:70–76. doi:10.1016/j.cbpc.2014.03.008
Li M, Gong S, Li Q, Yuan L, Meng F, Wang R (2016) Ammonia toxicity induces glutamine accumulation, oxidative stress and immunosuppression in juvenile yellow catfish Pelteobagrus fulvidraco. Comp Biochem Phys C Toxicol Pharmacol 183-184:1–6. doi:10.1016/j.cbpc.2016.01.005
Li M, Yu N, Qin JG, Li E, Du Z, Chen L (2014) Effects of ammonia stress, dietary linseed oil and Edwardsiella ictaluri challenge on juvenile darkbarbel catfish Pelteobagrus vachelli. Fish Shellfish Immun 38:158–165. doi:10.1016/j.fsi.2014.03.015
Livingstone DR (2003) Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Revue Méd Vét 154:427–430
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30. doi:10.1016/j.aquatox.2010.10.006
Lushchak VI (2014) Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 224:164–175. doi:10.1016/j.cbi.2014.10.016
Medeiros RS, Lopez BA, Sampaio LA, Romano LA, Rodrigues RV (2016) Ammonia and nitrite toxicity to false clownfish Amphiprion ocellaris. Aquacult Int 24:985–993. doi:10.1007/s10499-015-9965-9
Millner R, Walsh SJ, Díaz de Astarloa JM (2005) Atlantic flatfish fisheries. In: Gibson RN (ed) Flatfishes: biology and exploitation, 1st edn. Blackwell Science, Oxford, pp 240–271
Monserrat JM, Martínez PE, Geracitano LA, Amado LL, Martins CM, Pinho GL, Chaves IS, Ferreira-Cravo M, Ventura-Lima J, Bianchini A (2007) Pollution biomarkers in estuarine animals: critical review and new perspectives. Comp Biochem Phys C Toxicol Pharmacol 146:221–234. doi:10.1016/j.cbpc.2006.08.012
Oakes KD, Van Der Kraak GJ (2003) Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat Toxicol 63:447–463. doi:10.1016/S0166-445X(02)00204-7
Okamoto MH, Sampaio LA (2012) Sobrevivência e crescimento de juvenis do linguado Paralichthys orbignyanus criados em diferentes temperaturas. Atlântica (Rio Grande) 34:57–61. doi:10.5088/atlântica.v34i1.2710
Pan CH, Chien YH, Wang YJ (2011) Antioxidant defence to ammonia stress of characins (Hyphessobrycon eques Steindachner) fed diets supplemented with carotenoids. Aquac Nutr 17:258–266. doi:10.1111/j.1365-2095.2009.00747.x
Paust LO, Foss A, Imsland AK (2011) Effects of chronic and periodic exposure to ammonia on growth, food conversion efficiency and blood physiology in juvenile Atlantic halibut (Hippoglossus hippoglossus L.) Aquaculture 315:400–406. doi:10.1016/j.aquaculture.2011.03.008
Randall DJ, Tsui TK (2002) Ammonia toxicity in fish. Mar Pollut Bull 45:17–23. doi:10.1016/S0025-326X(02)00227-8
Rašković B, Jarić I, Koko V, Spasić M, Dulić Z, Marković Z, Poleksić V (2013) Histopathological indicators: a useful fish health monitoring tool in common carp (Cyprinus carpio Linnaeus, 1758) culture. Cent Eur J Biol 8:975–985. doi:10.2478/s11535-013-0220-y
Ravindrababu G, Neeraja P (2012) Histological changes in certain tissues of fish on ambient ammonia stress and post ammonia state (recovery). Int J Adv Biol Res 2:430–435
Rodrigues RV, Romano LA, Schwarz MH, Delbos B, Sampaio LA (2014) Acute tolerance and histopathological effects of ammonia on juvenile maroon clownfish Premnas biaculeatus (Block 1790). Aquac Res 45:1133–1139. doi:10.1111/are.12054
Roumieh R, Barakat A, Abdelmeguid NE, Ghanawi J, Saoud IP (2013) Acute and chronic effects of aqueous ammonia on marbled spinefoot rabbitfish, Siganus rivulatus (Forsskål 1775). Aquac Res 44:1777–1790. doi:10.1111/j.1365-2109.2012.03188.x
Sampaio LA, Bianchini A (2002) Salinity effects on osmoregulation and growth of the euryhaline flounder Paralichthys orbignyanus. J Exp Mar Biol Ecol 269:187–196. doi:10.1016/S0022-0981(01)00395-1
Sampaio LA, Freitas LS, Okamoto MH, Louzada LR, Rodrigues RV, Robaldo RB (2007) Effects of salinity on Brazilian flounder Paralichthys orbignyanus from fertilization to juvenile settlement. Aquaculture 262:340–346. doi:10.1016/j.aquaculture.2006.09.046
Sampaio LA, Robaldo RB, Bianchini A (2008) Hormone-induced ovulation, natural spawning and larviculture of Brazilian flounder Paralichthys orbignyanus (Valenciennes, 1839). Aquac Res 39:712–717. doi:10.1111/j.1365-2109.2008.01923.x
Schram E, Roques JA, Abbink W, Spanings T, de Vries P, Bierman S, van de Vis H, Flik G (2010) The impact of elevated water ammonia concentration on physiology, growth and feed intake of African catfish (Clarias gariepinus). Aquaculture 306:108–115. doi:10.1016/j.aquaculture.2010.06.005
Schreier HJ, Mirzoyan N, Saito K (2010) Microbial diversity of biological filters in recirculating aquaculture systems. Curr Opin Biotech 21:318–325. doi:10.1016/j.copbio.2010.03.011
Schwaiger J, Wanke R, Adam S, Pawert M, Honnen W, Triebskorn R (1997) The use of histopathological indicators to evaluate contaminant-related stress in fish. J Aquat Ecosyst Stress Recovery 6:75–86. doi:10.1023/A:1008212000208
Sevcikova M, Modra H, Blahova J, Dobsikova R, Plhalova L, Zitka O, Hynek D, Kizek R, Skoric M, Svobodova Z (2016) Biochemical, haematological and oxidative stress responses of common carp (Cyprinus carpio L.) after sub-chronic exposure to copper. Vet Med 61:35–50. doi:10.17221/8681-VETMED
Sinha AK, AbdElgawad H, Giblen T, Zinta G, De Rop M, Asard H, Blust R, De Boeck G (2014) Anti-oxidative defences are modulated differentially in three freshwater teleosts in response to ammonia-induced oxidative stress. PLoS One 9:e95319. doi:10.1371/journal.pone.0095319
Stoliar OB, Lushchak VI (2012) Environmental pollution and oxidative stress in fish. In: Lushchak VI (ed) Oxidative stress-environmental induction and dietary antioxidants. InTech. http://www.intechopen.com/books/oxidative-stress-environmental-induction-and-dietary-antioxidants/environmental-pollution-and-oxidative-stress-in-fish. Accessed 2 February 2017
Sun H, Wang W, Li J, Yang Z (2014) Growth, oxidative stress responses, and gene transcription of juvenile bighead carp (Hypophthalmichthys nobilis) under chronic-term exposure of ammonia. Environ Toxicol Chem 33:1726–1731. doi:10.1002/etc.2613
Velisek J, Stara A, Kolarova J, Svobodova Z (2011) Biochemical, physiological and morfological responses in common carp (Cyprinus carpio L.) after long-term exposure to terbutryn in real environmental concentration. Pestic Biochem Phys 100:305–313. doi:10.1016/j.pestbp.2011.05.004
Wasielesky W, Bianchini A, Miranda FK (1998) Tolerancia a la temperatura de juveniles de lenguado Paralichthys orbignyanus. Frente Marítimo 17:43–48
Wasielesky W, Bianchini A, Santos MH, Poersch LH (1997) Tolerance of juvenile flatfish Paralichthys orbignyanus to acid stress. J World Aquacult Soc 28:202–204. doi:10.1111/j.1749-7345.1997.tb00857.x
Wilhelm Filho D, Giulivi C, Boveris A (1993) Antioxidant defences in marine fish-I. Teleosts. Comp Biochem Phys C Pharmacol Toxicol Endocrinol 106:409–413. doi:10.1016/0742-8413(93)90154-D
Wilkie MP, Wood CM (1996) The adaptations of fish to extremely alkaline environments. Comp Biochem Phys B Biochem Mol Biol 113:665–673. doi:10.1016/0305-0491(95)02092-6
Yang W, Sun H, Xiang F, Yang Z, Chen Y (2011) Response of juvenile crucian carp (Carassius auratus) to long-term ammonia exposure: feeding, growth, and antioxidant defenses. J Freshw Ecol 26:563–570. doi:10.1080/02705060.2011.570944
Yang W, Xiang F, Liang L, Yang Z (2010a) Toxicity of ammonia and its effects on oxidative stress mechanisms of juvenile crucian carp (Carassius auratus). J Freshw Ecol 25:297–302. doi:10.1080/02705060.2010.9665080
Yang W, Xiang F, Sun H, Chen Y, Minter E, Yang Z (2010b) Changes in the selected hematological parameters and gill Na+/K+ ATPase activity of juvenile crucian carp Carassius auratus during elevated ammonia exposure and the post-exposure recovery. Biochem Syst Ecol 38:557–562. doi:10.1016/j.bse.2010.06.005
Zhang L, Feng L, Jiang WD, Liu Y, Jiang J, Li SH, Tang L, Kuang SY, Zhou XQ (2016) The impaired flesh quality by iron deficiency and excess is associated with increasing oxidative damage and decreasing antioxidant capacity in the muscle of young grass carp (Ctenopharyngodon idellus). Aquac Nutr 22:191–201. doi:10.1111/anu.12237
Zivna D, Plhalova L, Praskova E, Stepanova S, Siroka Z, Sevcikova M, Blahova J, Bartoskova M, Marsalek P, Skoric M, Svobodova Z (2013) Oxidative stress parameters in fish after subchronic exposure to acetylsalicylic acid. Neuroendocrinol Lett 34:116–122
Acknowledgements
This study was supported by research funds from the Conselho Nacional de Pesquisa e Desenvolvimento Científico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The authors are grateful to CNPq for productivity research fellowships provided for L. Garcia, L. Sampaio, L. Romano and J. Monserrat, and to CAPES for a PhD scholarship provided for MSc. Lucas C. Maltez.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Maltez, L.C., Stringhetta, G.R., Enamorado, A.D. et al. Ammonia exposure and subsequent recovery trigger oxidative stress responses in juveniles of Brazilian flounder Paralichthys orbignyanus . Fish Physiol Biochem 43, 1747–1759 (2017). https://doi.org/10.1007/s10695-017-0406-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-017-0406-8