Abstract
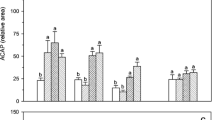
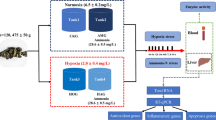
Catla catla catla (2.28 ± 0.1 g) were exposed to six different levels of dissolved oxygen: 1 (DO-1), 3 (DO-3), 5 (DO-5), 7 (DO-7), 9 (DO-9) and 11 (DO-11) mg/L. DO-5 served as control. In DO-1 and DO-3, the number of red blood cells (RBC), lysozyme, respiratory burst activity and nitric oxide synthase were significantly (p < 0.05) lower compared to the control one. In DO-7 and DO-9, RBC and lysozyme were significantly (p < 0.05) higher compared to the control one. Thiobarbituric acid reactive substances was significantly (p < 0.05) higher in catla exposed at low (1 and 3 mg/L) and high (9 and 11 mg/L) dissolved oxygen compared to others. In muscles and hepatopancreas, reduced glutathione was significantly (p < 0.05) higher in DO-5 and DO-7 and in gills of DO-5 compared to others after 1 h. In muscles, glutathione S-transferase (GST) was significantly (p < 0.05) lower in DO-5 and DO-7 compared to others. In hepatopancreas, GST and glutathione peroxidise (GPx) were significantly (p < 0.05) higher in DO-1 and DO-3 compared to others. In gills, GPx was higher in DO-9 and DO-11 after 48 h. In brain, hypoxia-inducible factor (HIF)-1α mRNA level was induced in DO-1 and DO-3 compared to others after 1 h of exposure. In gills and hepatopancreas, HIF-1α mRNA level was significantly (p < 0.05) higher in DO-1 compared to others after 1 h. The ATPase 6 mRNA level was significantly (p < 0.05) higher in brain and hepatopancreas of DO-1 after 1 h and in gills and hepatopancreas of DO-3 and DO-9, respectively, after 48 h compared to others.










Similar content being viewed by others
References
Alexander JB, Ingram GA (1992) Noncellular nonspecific defence mechanisms of fish. Annu Rev Fish Dis 2:249–279
Anderson DP, Siwicki AK (1995) Basic haematology and serology for fish health programs. In: Shariff M, Arthur JR, Subasinghe RP (eds) Diseases in Asian Aquaculture II. Manila, Fish Health Section, Asian Fisheries Society
Anderson S, Bankier AT, Barrell BG, de Bruijn MHL, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG (1981) Sequence and organization of the human mitochondrial genome. Nature 290:457–465
Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ (2009) An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res 37:4587–4602
Bhor VM, Raghuram N, Sivakami S (2004) Oxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin-induced diabetic rats. Int J Biochem Cell Biol 36:89–97
Bowden TJ (2008) Modulation of the immune system of fish by their environment. Fish Shellfish Immunol 25:373–383
Brander K (2010) Impacts of climate change on fisheries. J Mar Syst 79:389–402
Busk M, Boutilier RG (2005) Metabolic arrest and its regulation in anoxic eel hepatocytes. Physiol Biochem Zool 78:926–936
Coles BF, Kadlubar FF (2003) Detoxification of electrophilic compounds by glutathione S-transferase catalysis: determinants of individual response to chemical carcinogens and chemotherapeutic drugs? BioFactors 17:115–130
D’Avanzo C, Kremer JN (1994) Diel oxygen dynamics and anoxic events in an eutrophic estuary of Waquoit Bay, Massachusetts. Estuaries 17:131–139
Devasena T, Lalitha S, Padma K (2001) Lipid peroxidation, osmotic fragility and antioxidant status in children with acute post-streptococcal glomerulonephritis. Clin Chim Acta 308:155–161
Goel A, Dani V, Dhawan DK (2005) Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem Biol Interact 156:131–140
Gracey AY, Troll JV, Somero GN (2001) Hypoxia-induced gene expression profiling in the euryoxic fish Gillichthys mirabilis. Proc Natl Acad Sci USA 98:1993–1998
Graham JB (1990) Ecological, evolutionary, and physical factors influencing aquatic animal respiration. Am Zool 30:137–146
Grecay PA, Stierhoff KL (2002) A device for simultaneously controlling multiple treatment levels of dissolved oxygen in laboratory experiments. J Exp Mar Biol Ecol 280:53–62
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine, 2nd edn. Clarendon Press, Oxford
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Clarendon Press, Oxford
Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR (1998) Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. Proc Natl Acad Sci USA 95:5275–5280
Hermes-Lima M (2004) Oxygen in biology and biochemistry: role of free radicals. In: Storey KB (ed) Functional metabolism: regulation and adaptation. Wiley, New Jersey, pp 319–368
Hermes-Lima M, Zenteno-Savın T (2002) Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp Biochem Physiol C: Toxicol Pharmacol 133:537–556
Hochachka PW, Somero GN (2002) Biochemical adaptation: mechanism and process in physiological evolution, 1st edn. Oxford University Press, New York
Huttula T, Peltonen A, Bilaletdin Ä, Saura M (1992) The effects of climatic change on lake ice and water temperature. Aqua Fenn 22:129–142
Indian Council of Agricultural Research (2013) Handbook of Fisheries And aquaculture, 2nd edn. Indian Council of Agricultural Research, New Delhi, p 1116
Intergovernmental Panel on Climate Change (2007) Climate change 2007: the scientific basis. In: Solomon S et al (eds) Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, New York
Itoi S, Kinoshita S, Kikuchi K, Watabe S (2003) Changes of carp F0F1-ATPase in association with temperature acclimation. Am J Physiol Regul Integr Comp Physiol 284:R153–R163
Janssens BJ, Childress JJ, Baguet F, Rees J-F (2000) Reduced enzymatic antioxidative defense in deep-sea fish. J Exp Biol 203:3717–3725
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Jones OTG, Hancock JT, Henderson LM (1991) Oxygen radical production by transformed B lymphocytes. Mol Aspects Med 12:87–92
Koner BC, Banerjee BD, Ray A (1997) Effects of in vivo generation of oxygen free radicals on immune responsiveness in rabbits. Immunol Lett 59:127–131
Krumschnabel G, Schwarzbaum PJ, Biasi C, Dorigatti M, Wieser W (1997) Effects of energy limitation on Ca2+ and K+ homeostasis in anoxia-tolerant and anoxia-intolerant hepatocytes. Am J Physiol Regul Integr Comp Physiol 273:R307–R316
Kvamme BO, Gadan K, Finne-Fridell F, Niklasson L, Sundh H, Sundell K, Taranger GL, Evensen Ø (2013) Modulation of innate immune responses in Atlantic salmon by chronic hypoxia-induced stress. Fish Shellfish Immunol 34:55–65
Law SH, Wu RS, Ng PK, Richard MK, Kong RY (2006) Cloning and expression analysis of two distinct HIF-alpha isoforms—gcHIF-1alpha and gcHIF-4alpha—from the hypoxia-tolerant grass carp, Ctenopharyngodon idellus. BMC Mol Biol 7:15. doi:10.1186/1471-2199-7-15
Lee D-U, Kang YJ, Park MK, Lee YS, Seo HG, Kim TS, Kim CH, Chang KC (2003) Effects of 13-alkyl-substituted berberine alkaloids on the expression of COX-II, TNF-α, iNOS, and IL-12 production in LPS-stimulated macrophages. Life Sci 73:1401–1412
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Lushchak VI (2014) Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 224:164–175
Lushchak VI, Bagnyukova TV (2006) Effects of different environmental oxygen levels on free radical processes in fish. Comp Biochem Physiol B: Biochem Mol Biol 144:283–289
Lushchak VI, Lushchak LP, Mota AA, Hermes-Lima M (2001) Oxidative stress and antioxidant defenses in goldfish Carassius auratus during anoxia and reoxygenation. Am J Physiol Regul Integr Comp Physiol 280:R100–R107
Lushchak VI, Bagnyukova TV, Lushchak OV, Storey JM, Storey KB (2005a) Hypoxia and recovery perturb free radical processes and antioxidant potential in common carp (Cyprinus carpio) tissues. Int J Biochem Cell Biol 37:1319–1330
Lushchak VI, Bagnyukova TV, Husak VV, Luzhna LI, Lushchak OV, Storey KB (2005b) Hyperoxia results in transient oxidative stress and an adaptive response by antioxidant enzymes in goldfish tissues. Int J Biochem Cell Biol 37:1670–1680
Magnuson JJ, Crowder LB, Medvick PA (1979) Temperature as an ecological resource. Am Zool 19:331–343
Mallya YJ (2007) The effect of dissolved oxygen on fish growth in aquaculture. Kingolwira National Fish Farming Centre, Fisheries Division Ministry of Natural Resources and Tourism, Tanzania
Martinez-Alvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fisher 15:75–88
Martinovic D, Villeneuve DL, Kahl MD, Blake LS, Brodin JD, Ankley GT (2009) Hypoxia alters gene expression in the gonads of zebrafish (Danio rerio). Aquat Toxicol 95:258–272
Masella R, Di Benedetto R, Varì R, Filesi C, Giovannini C (2005) Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem 16:577–586
Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ (1984) Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney: possible implications in analgesic nephropathy. Biochem Pharmacol 33:1801–1807
Montgomery DC (1984) Design and analysis of experiments. Wiley, New York
Ni M, Wen H, Li J, Chi M, Bu Y, Ren Y, Zhang M, Song Z, Ding H (2014) The physiological performance and immune responses of juvenile Amur sturgeon (Acipenser schrenckii) to stocking density and hypoxia stress. Fish Shellfish Immunol 36:325–335
Oehlers LP, Perez AN, Walter RB (2007) Detection of hypoxia-related proteins in medaka (Oryzias latipes) brain tissue by difference gel electrophoresis and de novo sequencing of 4-sulfophenyl isothiocyanate-derivatized peptides by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Comp Biochem Physiol C: Toxicol Pharmacol 145:120–133
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Olsvik PA, Kristensen T, Waagbø R, Rosseland BO, Tollefsen KE, Baeverfjord G, Berntssen MHG (2005) mRNA expression of antioxidant enzymes (SOD, CAT and GSH-Px) and lipid peroxidative stress in liver of Atlantic salmon (Salmo salar) exposed to hyperoxic water during smoltification. Comp Biochem Physiol C: Toxicol Pharmacol 141:314–323
Padilla PA, Roth MB (2001) Oxygen deprivation causes suspended animation in the zebrafish embryo. Proc Natl Acad Sci USA 98:7331–7335
Pamplona R, Costantini D (2011) Molecular and structural antioxidant defenses against oxidative stress in animals. Am J Physiol Regul Integr Comp Physiol 301:R843–R863
Pompella A, Visvikis A, Paolicchi A, deTata V, Casini AF (2003) The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol 66:1499–1503
Rees BB, Sudradjat FA, Love JW (2001) Acclimation to hypoxia increases survival time of zebrafish, Danio rerio, during lethal hypoxia. J Exp Zool 289:266–272
Rissanen E, Tranberg HK, Sollid J, Nilsson GE, Nikinmaa M (2006) Temperature regulates hypoxia-inducible factor-1 (HIF-1) in a poikilothermic vertebrate, crucian carp (Carassius carassius). J Exp Biol 209:994–1003
Ritola O, Livingstone DR, Peters LD, Lindström-Seppä P (2002a) Antioxidant processes are affected in juvenile rainbow trout (Oncorhynchus mykiss) exposed to ozone and oxygen-supersaturated water. Aquaculture 210:1–19
Ritola O, Tossavainen K, Kiuru T, Lindstrom-Seppa P, Molsa H (2002b) Effects of continuous and episodic hyperoxia on stress and hepatic glutathione levels in one-summer-old rainbow trout (Oncorhynchus mykiss). J Appl Ichthyol 18:159–164
Rombout J, Huttenhuis HBT, Picchietti S, Scapigliati G (2005) Phylogeny and ontogeny of fish leucocytes. Fish Shellfish Immunol 19:441–455
Rytkönen KT, Prokkola JM, Salonen V, Nikinmaa M (2014) Transcriptional divergence of the duplicated hypoxia-inducible factor alpha genes in zebrafish. Gene 541:60–66
Salas-Leiton E, Cánovas-Conesa B, Zerolo R, López-Barea J, Cañavate JP, Albama J (2009) Proteomics of juvenile Senegal sole (Solea senegalensis) affected by gas bubble disease in hyperoxygenated ponds. Mar Biotechnol 11:473–487
Saurabh S, Sahoo PK (2008) Lysozyme: an important defence molecule of fish innate immune system. Aquac Res 39:223–239
Scapigliati G, Scalia D, Marras A, Meloni S, Mazzini M (1999) Immunoglobulin levels in the teleost sea bass Dicentrarchus labrax (L.) in relation to age, season, and water oxygenation. Aquaculture 174:207–212
Schrøder MB, Villena AJ, Jørgensen TØ (1998) Ontogeny of lymphoid organs and immunoglobulin producing cells in Atlantic cod (Gadus morhua L.). Dev Comp Immunol 22:507–517
Secor DH, Gunderson TE (1998) Effects of hypoxia and temperature on survival, growth, and respiration of juvenile Atlantic sturgeon, Acipenser oxyrinchus. Fish Bull 96:603–613
Shams I, Nevo E, Avivi A (2005) Ontogenetic expression of erythropoietin and hypoxia-inducible factor-1 alpha genes in subterranean blind mole rats. FASEB J 19:307–309
Sies H (1991) Role of reactive oxygen species in biological processes. Klin Wochenschr 69:965–968
Stierhoff KL, Targett TE, Grecay PA (2003) Hypoxia tolerance of the mummichog: the role of access to the water surface. J Fish Biol 63:580–592
Storey KB (1996) Oxidative stress: animal adaptations in nature. Braz J Med Biol Res 29:1715–1733
Ton C, Stamatiou D, Liew C-C (2003) Gene expression profile of zebrafish exposed to hypoxia during development. Physiol Genomics 13:97–106
Tripathi NK, Latimer KS, Burnley VV (2004) Hematologic reference intervals for koi (Cyprinus carpio), including blood cell morphology, cytochemistry, and ultrastructure. Vet Clin Pathol 33:74–83
Vig E, Nemcsok J (1989) The effects of hypoxia and paraquat on the superoxide dismutase activity in different organs of carp, Cyprinus carpio L. J Fish Biol 35:23–25
Watts M, Munday BL, Burke CM (2001) Immune responses of teleost fish. Aust Vet J 79:570–574
Wu Z, You F, Wen A, Ma D, Zhang P (2014) Physiological and morphological effects of severe hypoxia, hypoxia and hyperoxia in juvenile turbot (Scophthalmus maximus L.). Aquac Res. doi:10.1111/are.12483
Zhao S, Wu J (2013) Hypoxia inducible factor stabilization as a novel strategy to treat anemia. Curr Med Chem 20:2697–2711
Acknowledgments
Authors are thankful to Indian Council of Agricultural Research, ICAR, New Delhi, for providing financial support in the form of NFBSFARA project (AS-2001/2010-11) to carry out the research work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, S.P., Sharma, J., Ahmad, T. et al. Oxygen stress: impact on innate immune system, antioxidant defence system and expression of HIF-1α and ATPase 6 genes in Catla catla . Fish Physiol Biochem 42, 673–688 (2016). https://doi.org/10.1007/s10695-015-0168-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0168-0