Abstract
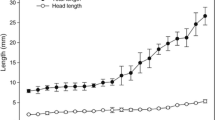
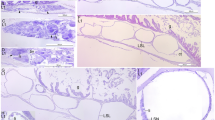
Biological aspects and global demand for aquarium promote seahorses as new species with high potential for commercial purposes; however, the low newborn survival rate represents the main bottleneck of seahorses farming. In this study, the organogenesis of the Hippocampus reidi was analysed from release until the 30th day after birth, using histological and histochemical approaches. To study the stages of their early life, 360 individuals were killed, sectioned, and stained with haematoxylin and eosin, periodic acid-Schiff, and Sudan Black B techniques. At birth, mouth and anus were open, the swim bladder inflated, and the visual system highly developed. Among the results, it was emphasized the presence of the yolk sac until the 2nd day after birth, the loops of the intestine to accommodate its elongation, and the ability of the larvae to absorb lipids in the anterior and posterior tract of the intestine. A short time (7/8 days) between reabsorption of yolk sac and formation of gonads was registered, with primordial follicles visible from the 10th day after birth. For the first time, organogenesis in H. reidi was described in detail; seahorses underwent a marked metamorphosis, and the indirect development observed in this species lead up to reconsider the term “juvenile” used for H. reidi during this period.







Similar content being viewed by others
References
Balon EK (1999) Alternative ways to become a juvenile or a definitive phenotype (and on some persisting linguistic offenses). Environ Biol Fish 56:17–38
Bishop CD, Erezyilmaz DF, Flatt T, Georgiou CD, Hadfield MG, Heyland A, Hodin J, Jacobs MW, Maslakova SA, Pires A, Reitzel AM, Santagata S, Tanaka K, Youson JH (2006) What is metamorphosis? Integr Comp Biol 46:655–661
Cañavate JP, Zerolo R, Fernandez-Diaz C (2006) Feeding and development of Senegal sole (Solea senegalensis) larvae reared in different photoperiods. Aquaculture 258:368–377
Celino FT, Hilomen-García GV, del Norte-Campos AG (2012) Feeding selectivity of the seahorse, Hippocampus kuda (Bleeker), juveniles under laboratory conditions. Aquac Res 43:1804–1815
Chatain B (1986) The swim bladder in Dicentrarchus labrax and Sparus aurata, 1: morphological aspects of development. Aquaculture 53:303–311
Chatain B (1994) Abnormal swimbladder development and lordosis in sea bass (Dicentrarchus labrax) and sea bream (Sparus auratus). Aquaculture 119:371–379
Chatain B, Dewavrin G (1989) The effect of abnormalities in the development of the swim bladder on the mortality of Dicentrarchus labrax during weaning. Aquaculture 78:55–61
Choo CK, Liew HC (2006) Morphological development and allometric growth patterns in the juvenile seahorse Hippocampus kuda Bleeker. J Fish Biol 69:426–445
CITES (2002) Convention on international trade in endangered species of wild fauna and Flora. Twelfth Meeting of the Conference of the Parties, Santiago de Chile (CoP12 Doc. 43, p 20), Chile, 3–15 November. http://www.cites.org/eng/cop/12/doc/E12-43.pdf
Cuvier G (1817) Le règne animal distribué d’après son organisation: Les reptiles, les poissons, les mollusques, et les annélides. Tome II. Paris, Chez Déterville
Deplano M, Diaz J, Connes R, Kentouri-Divanach M, Cavalier F (1991) Appearance of lipid-absorption capacities in larvae of the sea bass Dicentrarchus labrax during transition to the exotrophic phase. Mar Biol 108:361–371
Easter S (1992) Retinal growth in foveated teleosts: nasotemporal asymmetry keeps the fovea in temporal retina. J Neurosci 12:2381–2392
Edwards JG, Condorelli L (1928) Studies on aglomerular and glomerular kidneys II. Physiological. Am J Physiol 86:383–398
Einarsdóttir IE, Silva N, Power DM, Smáradóttir H, Björnsson BT (2006) Thyroid and pituitary gland development from hatching through metamorphosis of a teleost flatfish, the Atlantic halibut. Anat Embryol 211:47–60
Fishelson L, Delarea Y (2004) Taste buds on the lips and mouth of some blenniid and gobiid fishes: comparative distribution and morphology. J Fish Biol 65:651–665
Foster S, Vincent A (2004) Life history and ecology of seahorses: implications for conservation and management. J Fish Biol 65:1–61
García-Hernández M, Lozano M, Elbal M, Agulleiro B (2001) Development of the digestive tract of sea bass (Dicentrarchus labrax L). Light and electron microscopic studies. Anat Embryol 204:39–57
Gisbert E, Piedrahita RH, Conklin DE (2004) Ontogenetic development of the digestive system in California halibut (Paralichthys californicus) with notes on feeding practices. Aquaculture 232:455–470
Govoni JJ, Boehlert GW, Watanabe Y (1986) The physiology of digestion in fish larvae. Environ Biol Fish 16:59–77
Hachero-Cruzado I, Ortiz-Delgado JB, Borrega B, Herrera M, Navas J, Sarasquete C (2009) Larval organogenesis of flatfish brill Scophthalmus rhombus L.: histological and histochemical aspects. Aquaculture 286:138–149
Hansen A, Reutter K, Zeiske E (2002) Taste bud development in the zebrafish, Danio rerio. Dev Dyn 223:483–496
Hickman C, Trump B (1969) The kidney. In: Hoar WS, Randall DJ (eds) Fish physiology. I. Academic Press, New York, pp 91–239
Hilomen-García G, De los Reyes R, García C (2003) Tolerance of seahorse Hippocampus kuda (Bleeker) juveniles to various salinities. J Appl Ichthyol 19:94–98
Hopkins KD (1992) Reporting fish growth: a review of the basics. J World Aquac Soc 23:173–179
Hora MSC, Joyeux JC (2009) Closing the reproductive cycle: growth of the seahorse Hippocampus reidi (Teleostei, Syngnathidae) from birth to adulthood under experimental conditions. Aquaculture 292:37–41
Ingram K (2005) Farming seahorses, an environmental saviour. NZ Aquac 7:6–8
Jaroszewska M, Dabrowski K (2011) Utilization of yolk: transition from endogenous to exogenous nutrition in fish. In: Holt GJ (ed) Larval fish nutrition. Wiley-Blackwell, Oxford, pp 183–218
Kjørsvik E, Meeren T, Kryvi H, Arnfinnson J, Kvenseth P (1991) Early development of the digestive tract of cod larvae, Gadus morhua L., during start-feeding and starvation. J Fish Biol 38:1–15
Lee HR, O’brien KMB (2011) Morphological and behavioral limit of visual resolution in temperate (Hippocampus abdominalis) and tropical (Hippocampus taeniopterus) seahorses. Vis Neurosci 28:351–360
Lin Q, Li G, Qin G, Lin J, Huang L, Sun H, Feng P (2012) The dynamics of reproductive rate, offspring survivorship and growth in the lined seahorse, Hippocampus erectus Perry, 1810. Biol Open 1:391–396
Lourie S (2003) Measuring seahorses. Project Seahorse Technical Report No. 4, Version 1.0. Project Seahorse, Fisheries Centre, University of British Columbia
Maack G, Segner H (2003) Morphological development of the gonads in zebrafish. J Fish Biol 62:895–906
Mai ACG, Velasco G (2012) Population dynamics and reproduction of wild longsnout seahorse Hippocampus reidi. J Mar Biol Assoc 92:421–427
Martins M, Mouriño J, Fezer G, Buglione Neto C, Garcia P, Silva B, Jatobá A, Vieira F (2010) Isolation and experimental infection with Vibrio alginolyticus in the sea horse, Hippocampus reidi Ginsburg, 1933 (Osteichthyes: Syngnathidae) in Brazil. Braz J Biol 70:205–209
Martoja R, Martoja-Pierson M, Grassé PP, Moncanut ME, Coll MD (1970) Técnicas de histología animal. Toray-Masson Barcelona
Miyazaki T, Fujiwara K (1988) Histological studies on yolk utilization and digestive function in larvae and juvenile of red sea bream and black sea bream. Bull Fac Bioresour Mie Univ 1:15–27
Mosk V, Thomas N, Hart NS, Partridge JC, Beazley LD, Shand J (2007) Spectral sensitivities of the seahorses Hippocampus subelongatus and Hippocampus barbouri and the pipefish Stigmatopora argus. Vis Neurosci 24:345–354
Murugan A, Dhanya S, Sreepada RA, Rajagopal S, Balasubramanian T (2009) Breeding and mass-scale rearing of three spotted seahorse, Hippocampus trimaculatus Leach under captive conditions. Aquaculture 290:87–96
Nakanishi T (1986) Seasonal changes in the humoral immune response and the lymphoid tissues of the marine teleost, Sebastiscus marmoratus. Vet Immunol Immunopathol 12:213–221
Ortiz-Delgado JB, Iglesias J, Sánchez FJ, Cal R, Lago MJ, Otero JJ, Sarasquete C (2011) A morphohistological and histochemical study of hatchery-reared European hake, Merluccius merluccius (Linnaeus, 1758), during the lecitho-exotrophic larval phase. Sci Mar 76:259–271
Otero-Ferrer F, Molina L, Socorro J, Herrera R, Fernández-Palacios H, Izquierdo MS (2010) Live prey first feeding regimes for short-snouted seahorse Hippocampus hippocampus (Linnaeus, 1758) juveniles. Aquac Res 41:8–19
Otero-Ferrer F, Izquierdo M, Segade A, Fazeli A, Holt W (2013) Seahorses: a new epigenetic model. In: Proceedings of the EPICONCEPT Workshop 2013 Epigenetics for improved food production: from model to practice. ISBN 978-84-9965-181-1
Otero-Ferrer F, Izquierdo M, Fazeli A, Holt W (2014) Embryonic developmental plasticity in the long-snouted seahorse (Hippocampus reidi, Ginsburg 1933) in relation to parental preconception diet. Reprod Fertil Dev. doi:10.1071/RD14169
Palma J, Bureau DP, Andrade JP (2014) The effect of diet on ontogenic development of the digestive tract in juvenile reared long snout seahorse Hippocampus guttulatus. Fish Physiol Bioch 40:739–750
Papadakis IE, Kentouri M, Divanach P, Mylonas CC (2013) Ontogeny of the digestive system of meagre Argyrosomus regius reared in a mesocosm, and quantitative changes of lipids in the liver from hatching to juvenile. Aquaculture 388:76–88
Pham NK, Lin J (2013) The effects of different feed enrichments on survivorship and growth of early juvenile longsnout seahorse, Hippocampus reidi. J World Aquac Soc 44:435–446
Power DM, Llewellyn L, Faustino M, Nowell MA, Björnsson BT, Einarsdottir IE, Canario AVM, Sweeney GE (2001) Thyroid hormones in growth and development of fish. Comp Biochem Physiol Part C Comp Pharmacol Toxicol 130:447–459
Ricker WE (1958) Handbook of computations for biological statistics of fish populations. Fish Res Board Can, Ottawa
Roo J, Hernandez-Cruz CM, Borrero C, Schuchardt D, Fernandez-Palacios H (2010) Effect of larval density and feeding sequence on meagre (Argyrosomus regius; Asso, 1801) larval rearing. Aquaculture 302:82–88
Rosa IL, Dias TL, Baum JK (2002) Threatened fishes of the world: Hippocampus reidi Ginsburg, 1933 (Syngnathidae). Environ Biol Fish 64:378
Rosa IL, Oliveira TP, Osório FM, Moraes LE, Castro AL, Barros GM, Alves RR (2011) Fisheries and trade of seahorses in Brazil: historical perspective, current trends, and future directions. Biodivers Conserv 20:1951–1971
Santamaría C, Marín de Mateo M, Traveset R, Sala R, Grau A, Pastor E, Sarasquete C, Crespo S (2004) Larval organogenesis in common dentex Dentex dentex L. (Sparidae): histological and histochemical aspects. Aquaculture 237:207–228
Sarasquete M, Polo A, De Canales MG (1993) A histochemical and immunohistochemical study of digestive enzymes and hormones during the larval development of the sea bream, Sparus aurata L. Histochem J 25:430–437
Sarasquete M, Polo A, Yúfera M (1995) Histology and histochemistry of the development of the digestive system of larval gilthead seabream, Sparus aurata L. Aquaculture 130:79–92
Schrøder MB, Villena AJ, Jorgensen TO (1998) Ontogeny of lymphoid organs and immunoglobulin producing cells in Atlantic cod (Gadus morhua L.). Dev Comp Immunol 22:507–517
Silveira RB (2000) Desenvolvimento osteólogico de Hippocampus reidi Ginsburg (Pisces, Syngnathiformes, Syngnathidae) em laboratorio. II. Periódo juveníl. Rev Bras Zool 17:515–531
Socorro JA (2006) Estudio comparado del desarrollo embrionario y larvario del bocinegro (Pagrus pagrus) y de la sama de pluma (Dentex gibbosus). PhD Thesis. University of Las Palmas de Gran Canaria
Storero LP, González RA (2009) Prey selectivity and trophic behavior of the Patagonian seahorse, Hippocampus patagonicus, in captivity. J World Aquac Soc 40:394–401
Van Wassenbergh S, Roos G, Genbrugge A, Leysen H, Aerts P, Adriaens D, Herrel A (2009) Suction is kid’s play: extremely fast suction in newborn seahorses. Biol Lett 5:200–203
Vincent ACJ (1996) The international trade in seahorse. TRAFFIC Int, Cambridge
Watanabe Y (1982) Intracellular digestion of horseradish peroxidase (as a marker protein) by the intestinal cells of teleost larvae and juveniles. Bull Jpn Soc Sci Fish 48:37–42
Watanabe T, Kitajima C, Fujita S (1983) Nutritional value of live organisms used in Japan for mass propagation of fish: a review. Aquaculture 34:115–143
Whitfield AK (1995) Threatened fishes of the world: Hippocampus capensis Boulenger, 1900 (Syngnathidae). Environ Biol Fish 44:362
Willadino L, Souza-Santos LP, Mélo R, Brito AP, Barros N, Araújo-Castro C, Galvão DB, Gouveia A, Regis CG, Cavalli RO (2012) Ingestion rate, survival and growth of newly released seahorse Hippocampus reidi fed exclusively on cultured live food items. Aquaculture 360:10–16
Wold PA, Hoehne-Reitan K, Cahu CL, Zambonino Infante JL, Rainuzzo J, Kjørsvik E (2007) Phospholipids vs. neutral lipids: effects on digestive enzymes in Atlantic cod (Gadus morhua) larvae. Aquaculture 272:502–513
Woods C (2000) Improving initial survival in cultured seahorses, Hippocampus abdominalis Leeson, 1827 (Teleostei: Syngnathidae). Aquaculture 190:377–388
Yúfera M, Darias MJ (2007) The onset of exogenous feeding in marine fish larvae. Aquaculture 268:53–63
Zapata A, Díez B, Cejalvo T, Gutiérrez-de Frías C, Cortés A (2006) Ontogeny of the immune system of fish. Fish Shellfish Immunol 20:126–136
Acknowledgments
In memoriam of Professor Massimo Trentini. The authors thank the Parque Científico Tecnológico Marino (PCTM) of the University of Las Palmas de Gran Canaria (ULPGC), and the Instituto Universitario de Sanidad Animal y Seguridad Alimentaria (IUSA) for support this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Novelli, B., Socorro, J.A., Caballero, M.J. et al. Development of seahorse (Hippocampus reidi, Ginsburg 1933): histological and histochemical study. Fish Physiol Biochem 41, 1233–1251 (2015). https://doi.org/10.1007/s10695-015-0082-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0082-5