Abstract
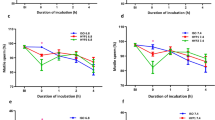
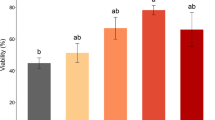
The role of environmental ion composition and osmolality in Ca2+ signaled activation was assessed in spermatozoa of brook trout Salvelinus fontinalis. Milt from ten mature males was obtained by abdominal massage. Spermatozoa motility was evaluated in 0, 100, and 300 mOsm/kg NaCl or sucrose solutions, buffered by 10 mM Tris–HCl pH 8.5. For investigation of spermatozoa reaction to external Ca2+ concentration, 2 mM ethylene glycol tetraacetic acid (EGTA) was added to the activation media as a calcium ions chelator. For investigation of the effect of external Na+ concentration in conditions of low external Ca2+, 100 µM amiloride was added to the EGTA-containing solutions as a Na+ transport blocker. Low motility was observed in sucrose (Na+ free) solutions containing 2 mM EGTA but not in Na+ solutions containing 2 mM EGTA. Addition of amiloride led to significantly increased motility (P < 0.05) compared with sucrose (Na+ free) solutions containing 2 mM EGTA. We conclude that Na+ transport in Ca2+-free solutions plays a regulatory role in brook trout spermatozoa activation. The influence of competitive Na+ and Ca2+ transport on the control of spermatozoa activation requires further study with respect to its application for improvement of artificial activation and storage media.

Similar content being viewed by others
References
Alavi SMH, Cosson J (2005) Sperm motility in fishes: (I) effects of temperature and pH: a review. Cell Biol Int 29:101–110
Alavi SMH, Cosson J (2006) Sperm motility in fishes: (II) effects of ions and osmotic pressure: a review. Cell Biol Int 30:1–14
Alavi SMH, Gela D, Rodina M, Linhart O (2011) Roles of osmolality, calcium-potassium antagonist and calcium in activation and flagellar beating pattern of sturgeon. Comp Biochem Physiol A 160:166–174
Baynes SM, Scott AP, Dawson AP (1981) Rainbow trout, Salmo gairdneri, spermatozoa: effects of cations and pH on motility. J Fish Biol 19:259–267
Boitano MP, Omoto CK (1991) Membrane hyperpolarization activates trout sperm without an increase in intracellular pH. J Cell Sci 98:343–349
Cosson J (2004) The ionic and factors controlling motility of fish spermatozoa. Aquac Int 12:69–85
Cosson J (2010) Frenetic activation of fish spermatozoa flagella entails short-term motility, portending their precocious decadence. J Fish Biol 76:240–279
Cosson MP, Billard R, Letellier L (1989) Rise on internal Ca2+ accompanies the initiation of trout sperm motility. Cell Motil Cytoskeleton 14:424–434
Cosson J, Billard R, Gibert C, Dreanno C, Suquet M (1999) Ionic factors regulating the motility of fish sperm. In: Gagnon C (ed) The male gamete: from basic to clinical applications. Cache River Press, Vienna, pp 161–186
Darszon A, Labarca P, Nishigaki T, Espinosa P (1999) Ion channels in sperm physiology. Physiol Rev 79(2):481–502
Darszon A, Beltran C, Felix R, Nishigaki Trevino CL (2001) Ion transport in sperm signaling. Dev Biol 240:1–14
Kho KH, Tanimoto S, Inaba K, Oka Y, Morisawa M (2001) Transmembrane cell signaling for the initiation of trout sperm motility: roles of ion channels and membrane hyperpolarization for cAMP synthesis. Zool Sci 18:919–928
Krasznai Z, Marian T, Isumi H, Damjanovich S, Balkay L, Tron L, Morisawa M (1999) Membrane hyperpolarization removes inactivation of Ca2+ channels, leading Ca2+ influx subsequent initiation of sperm motility in common carp. PNAS 97:2052–2057
Krasznai Z, Morisawa M, Krasznai ZT, Morisawa S, Inaba K, Bazsane ZK, Rubovszky B, Bodnar B, Borsos A, Marian T (2003) Gadolinium, a mechano-sensitive channel blocker, inhibits osmosis-initiated motility of sea- and freshwater fish sperm, but does not affect human or ascidian sperm motility. Cell Motil Cytoskeleton 55:232–243
Li P, Li ZH, Hulak M, Rodina M, Linhart O (2012) Regulation of spermatozoa motility in response to cations in Russian sturgeon Acipenser gueldenstaedtii. Theriogenology 78:102–109
Linhart O, Rodina M, Cosson J (2000) Cryopreservation of sperm in common carp Cyprinus carpio: sperm motility and hatching success of embryos. Cryobiology 41:241–250
Mogas MT, Rivera del Alamo M, Rodriguez-Gil JE (2010) Roles of Na-/K–dependent ATPase, Na-/H- antiporter and GLUT hexose transporters in the cryosurvival of dog spermatozoa: effects on viability, acrosome state and motile sperm subpopulation structure. Theriogenology 75:1669–1681
Morisawa M (2008) Adaptation and strategy for fertilization in the sperm of teleost fish. J Appl Ichthyol 24:362–370
Morisawa M, Suzuki K (1980) Osmolality and potassium ion: their roles in initiation of sperm motility in teleosts. Science 210:1145–1147
Morita M, Fujinoki M, Okuno M (2005) K+-independent initiation of motility in chum salmon sperm treated with an organic alcohol, glycerol. J Exp Biol 208:4549–4556
Morsawa M, Susuki K, Shimizu H, Morisawa S, Yasuda K (1983) Effect of osmolality and potassium on motility of spermatozoa from freshwater cyprinid fishes. J Exp Zool 107:95–103
Padan E, Venturi M, Gerchman Y, Dover N (2001) Na−/H− antiporters. Biochim Biophys Acta 1505:144–157
Siegel S, Castellan NJ (1988) Nonparametric statistics for the behavioral sciences, 2nd edn. McGraw-Hill, New York
Takei GL, Mukai C, Okuno M (2011) Transient Ca2+ mobilization caused by osmotic shock initiates salmonid fish sperm motility. J Exp Biol 215:630–641
Acknowledgments
We are grateful to Lucidus Consultancy, UK for English correction and suggestions. This work was supported by several research grants such as CENAKVA CZ.1.05/2.1.00/01.0024, strengthening of excellence scientific teams in USB FFPW CZ.1.07/2.3.00/20.0024, GAJU 114/2013/Z, GAJU 123/2014/Z, GACR P502/11/0090, and GACR P502/12/1973. The results of the project LO1205 were obtained with a financial support from the MEYS of the CR under the NPU I program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bondarenko, O., Dzyuba, B., Cosson, J. et al. The role of Ca2+ and Na+ membrane transport in brook trout (Salvelinus fontinalis) spermatozoa motility. Fish Physiol Biochem 40, 1417–1421 (2014). https://doi.org/10.1007/s10695-014-9936-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-014-9936-5