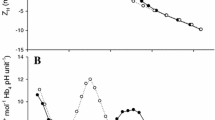
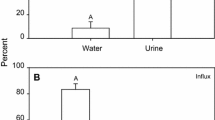
Abstract
The physiological relevance of the teleost pseudobranch as a remnant of a reduced gill arch is still unclear. Numerous hypotheses have been proposed regarding its physiological role, but direct confirmatory evidence is lacking. The close relationship by serial blood flow arrangement with the fish eye’s choroid rete has sparked the idea that pseudobranchial preconditioning of blood pH may facilitate initiation of the Root effect and thus support the establishment of high oxygen tensions for retinal diffusive supply. This idea was critically tested by studies on isolated pseudobranchs in situ (Oncorhynchus mykiss), perfused with RBC/Ringer or RBC/plasma suspensions of widely varied composition (pH 7.4–8.2). Detailed analysis of inflowing as compared to effluent perfusates indicated normal aerobic metabolism expressed by a rise in Pco2 (+0.39 ± 0.13 mmHg \( \overline{{\text{x}}} {\text{ $ \pm $ SD}} \)), an oxygen utilization of 25% and a high oxygen consumption of ∼400 nmol g−1 min−1. Upon passage of the pseudobranch, pH (corrected for Haldane effect) was only slightly acidified (−0.03 to −0.10), [HCO3 −] and [lactate] were slightly enhanced (+0.51 mmol l−1 or 0.13 mmol l−1, respectively). In order to test for yet unknown plasma components involved in pseudobranch function, a second series of experiments was conducted using RBC-suspensions in fresh plasma instead of Ringer, with results closely resembling those of the RBC/Ringer series. Lacking any physiologically significant correlation with the level of perfusate pH, the obtained data indicate pseudobranchial basic metabolic activity rather than pH regulatory characteristics. Also the observed absolute changes in pH are negligible in terms of pH regulation towards the Root-threshold. Accordingly, the present experiments as well as plausibility evaluation of mechanisms do not support the idea of blood pH pre-adjustment prior to entry into the choroid rete structure of the teleost eye to facilitate the Root-mediated oxygen release.




Similar content being viewed by others
References
Bamford OS (1974) Oxygen reception in the rainbow trout (Salmo gairdneri). Comp Biochem Physiol A 48:69–76
Benadé AJS, Heisler N (1978) Comparison of efflux rates of hydrogen and lactate ions from isolated muscles in vitro. Respir Physiol 32:369–380
Berenbrink M (1995) Die Kontrolle des intrazellulären pH in den Erythrozyten von Knochenfischen. PhD thesis, Heinrich-Heine-Universität Düsseldorf, Germany
Bridges CR (1983) Po2 and oxygen content measurement in blood samples using polarographic oxygen sensors. In: Gnaiger E, Forstner H (eds) Polarographic oxygen sensors. Springer, Berlin, pp 219–233
Bridges CR, Berenbrink M, Müller R, Waser W (1998) Physiology and biochemistry of the pseudobranch: An unanswered question? Comp Biochem Physiol A 119:67–77
Copeland DE (1947) The interrelation of the chloride excreting cell (gill) and the pseudobranch of Fundulus heteroclitus. Biol Bull 93:222
Dendy LA, Deter RL, Philpott CW (1973a) Localization of Na+, K+-ATPase and other enzymes in teleost pseudobranch. I. Biochemical characterization of subcellular fractions. J Cell Biol 57:675–688
Dendy LA, Philpott CW, Deter RL (1973b) Localization of Na+, K+-ATPase and other enzymes in teleost pseudobranch. II. Morphological characterization of intact pseudobranch, subcellular fractions and plasma membrane structure. J Cell Biol 57:689–703
Drabkin DL (1965) The molecular weight of haemoglobin, its iron and nitrogen content and optical properties – their relevance in the problem of a reference standard for haemoglobin measurement. Bibl Haematol 21:35–42
Drabkin DL, Austin JH (1935) Spectrophotometric studies: II. Preparations from washed blood cells; nitric oxide hemoglobin and sulfhemoglobin. J Biol Chem 112:51–65
Farhi L (1965) Continuous duty tonometer system. J Appl Physiol 20:1098–1101
Fairbanks MB, Hoffert JR, Fromm PO (1969) The dependance of the oxygen-concentrating mechanism of the teleost eye (Salmo gairdneri) on the enzyme carbonic anhydrase. J Gen Physiol 54:203–211
Fairbanks MB, Hoffert JR, Fromm PO (1974) Short circuiting of the ocular oxygen concentrating mechanism in the teleost Salmo gairdneri using carbonic anhydrase inhibitors. J Gen Physiol 64:263–273
Heisler N (1986a) Buffering and transmembrane ion transfer processes. In: Heisler N (ed) Acid-base regulation in animals. Elsevier, Amsterdam, pp 397–450
Heisler N (1986b) Acid-base regulation in fishes. In: Heisler N (ed) Acid-base regulation in animals. Elsevier, Amsterdam, pp 397–450
Heisler N (1986c) Comparative aspects of acid-base regulation. In: Heisler N (ed) Acid-base regulation in animals. Elsevier, Amsterdam, pp 397–450
Heisler N (2004) Buffering and H+ dynamics in muscle tissues. Respir Physiol Neurobiol 144:161–172
Hoffert JR (1966) Observations on ocular fluid dynamics and carbonic anhydrase in tissues of lake trout (Salvelinus namaycush). Comp Biochem Physiol 17:107–114
Holeton G, Randall DJ (1967) Changes of blood pressure in the rainbow trout during hypoxia. J Exp Biol 46:297–305
Ishimatsu A, Iwama GK, Heisler N (1988) In vivo analysis of the partitioning of cardiac output between systemic and central venous sinus circuits in rainbow trout: a new approach using chronic cannulation of the branchial vein. J Exp Biol 137:75–88
Iwama GK, Ishimatsu A, Heisler N (1993) Site of acid-base relevant ion transfer in the gills of rainbow trout (Oncorhynchus mykiss) exposed to environmental hypercapnia. Fish Physiol Biochem 12:269–280
Jensen FB (1989) Hydrogen ion equilibria in fish haemoglobins. J Exp Biol 143:225–234
Kern G, Bösch ST, Unterhuber E, Pelster B (2002) Mechanisms of acid secretion in pseudobranch cells of rainbow trout Oncorhynchus mykiss. J Exp Biol 205:2943–2954
Kryzanovsky SG (1934) Die Pseudobranchie. Morphologie und biologische Bedeutung. Zool Jahrb Abt Anat Ontog Tiere 58:171–238
Laurent P (1974) Pseudobranchial receptors in teleosts. In: Autrum H (ed) Handbook of sensory physiology, vol 3,3: Electroceptors and other specialized receptors in lower vertebrates Fessard A, Bullock TH, Autrum H (eds) Springer, Berlin, pp 279–296
Laurent P, Dunel-Erb S (1984) The pseudobranch: morphology and function. In: Hoar WS, Randall DJ (eds) Fish physiology, vol Xb: gills. Ion and water transfer. Academic Press, Orlando, pp 285–323
Laurent P, Rouzeau J-D (1972) Afferent neural activity from pseudobranch of teleosts. Effects of PO2, pH, osmotic pressure and Na+ ions. Respir Physiol 14:307–331
Laurent P, Dunel S, Barets A (1969) Localisation histochimique de l’anhydrase carbonique au niveau des chemorécepteurs artériels das Mammifères, des Batraciens et des Poissons. Histochemie 17:99–107
Leiner M (1938) Die Augenkiemendrüse (Pseudobranchie) der Knochenfische. Experimentelle Untersuchungen über ihre physiologische Bedeutung. Z Vergl Physiol 26:416–466
Leiner M (1940) Das Atmungsferment Kohlensäureanhydrase im Tierkörper. Naturwissenschaften 28:165–171
Maetz J (1956) Le rôle biologique de l’anhydrase carbonique chez quelques Téléostéens. Bull Biol Fr Belg 40:1–129
Morgan JD, Iwama GK (1999) Energy cost of NaCl transport in isolated gills of cutthroat trout. Am J Physiol 277:R631–R639
Müller J (1839) Vergleichende Anatomie der Myxinoiden. III. Über das Gefäßsystem. Abh Dtsch Akad Wiss Berlin 175–303
Müller R (1995) Untersuchungen zur Funktion der Pseudobranchie bei Teleosteern. Masters Thesis, Heinrich-Heine-Universität, Düsseldorf, Germany
Nikinmaa M, Tiihonen K, Paajaste M (1990) Adrenergic control of red cell pH in salmonid fish: roles of the sodium/proton exchange, Jacobs-Stewart cycle and membrane potential. J Exp Biol 154:257–271
Parry G, Holliday FGT (1960) An experimental analysis of the function of the pseudobranch in teleosts. J Exp Biol 37:344–353
Pelster B, Kobayashi H, Scheid P (1989) Metabolism of the perfused swimbladder of the European eel: oxygen, carbon dioxide, glucose and lactate balance. J Exp Biol 144:495–506
Perry SF, Heming TA (1981) Blood ionic and acid-base status in rainbow trout (Salmo gairdneri) following rapid transfer from freshwater to seawater: effect of pseudobranch denervation. Can J Zool 59:1126–1132
Randall DJ, Jones DR (1973) The effect of deafferentation of the pseudobranch on the response to hypoxia and hyperoxia in the trout (Salmo gairdneri). Respir Physiol 17:291–301
Randall DJ, Smith LS, Brett JR (1965) Dorsal aortic blood pressure recorded from rainbow trout (Salmo gaidneri). Can J Zool 43:863–877
Smith FM, Jones DR (1978) Localization of receptors causing hypoxic bradycardia in trout (Salmo gairdneri). Can J Zool 56:1260–1265
Sobotka H, Kann S (1941) Carbonic anhydrase in fish and invertebrates. J Cell Comp Physiol 17:341–348
Stevens ED, Randall DJ (1967a) Changes in blood pressure, heart rate and breathing rate during moderate swimming activity in rainbow trout. J Exp Biol 46:307–315
Stevens ED, Randall DJ (1967b) Changes of gas concentrations in blood and water during moderate swimming activity in rainbow trout. J Exp Biol 46:329–337
Tetens V, Christensen NJ (1987) Beta-adrenergic control of blood oxygen affinity in acutely hypoxia exposed rainbow trout. J Comp Physiol B 157:667–675
Thomas R (1989) Bicarbonate and pHi response. Nature 337:601
Tufts BL, Boutilier RG (1991) Interactions between ion exchange and metabolism in erythrocytes of the rainbow trout (Oncorhynchus mykiss). J Exp Biol 156:139–151
Vogel WOP (1985) Systemic vascular anastomoses, primary and secondary vessels in fish, and the phylogeny of lymphatics. In: Johansen K, Burggren WW (eds) Cardiovascular shunts; phylogenetic, ontogenetic and clinical aspects. Munksgaard, Copenhagen, pp 143–151
Waser WP, Heisler N (2004) Oxygen delivery to the fish eye: blood flow in the pseudobranchial artery of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 30:77–85
Waser W, Heisler N (2005) Oxygen delivery to the fish eye: root effect as a crucial factor for elevated retinal PO2. J Exp Biol 208:4035–4047
Waser WP, Schmitz S, Perry SF, Wobschall A (2005) Stereological analysis of blood space and tissue types in the pseudobranch of the rainbow trout (Oncovhynchus mykiss). Fish Physiol Biochem 31:73–82
Weber RE, Jensen FB, Cox RP (1987) Analysis of teleost hemoglobin by Adair and Monod-Wyman-Changeux models. J Comp Physiol B 157:145–152
Wilkie MP, Wood CM (1995) Recovery from high pH exposure in the rainbow trout: white muscle ammonia storage, ammonia washout, and the restoration of blood chemistry. Physiol Zool 68:379–401
Wittenberg JB, Haedrich RL (1974) The choroid rete mirabile of the fish eye. II. Distribution and relation to the pseudobranch and to the swimbladder rete mirabile. Biol Bull 146:137–156
Wittenberg JB, Wittenberg BA (1962) Active secretion of oxygen into the eye of fish. Nature 194:106–107
Wittenberg JB, Wittenberg BA (1974) The choroid rete mirabile of the fish eye. I. Oxygen secretion and structure: comparison with the swimbladder rete mirabile. Biol Bull 146:116–136
Wood CM, Pärt P (2000) Intracellular pH regulation and buffer capacity in CO2/HCO3 − buffered media in cultured epithelial cells from rainbow trout gills. J Comp Physiol B 170:175–184
Acknowledgement
We would like to thank Brigitte Geue for her invaluable assistance during experiments and analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mölich, A., Waser, W. & Heisler, N. The teleost pseudobranch: a role for preconditioning of ocular blood supply?. Fish Physiol Biochem 35, 273–286 (2009). https://doi.org/10.1007/s10695-008-9207-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-008-9207-4