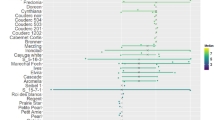
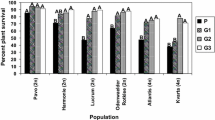
Abstract
Red clover (Trifolium pratense) is an important perennial forage crop that is widely cultivated in Europe. Clover rot remains a major disease in red clover, but resistance breeding is hampered by the lack of available sources of resistance. Moreover, little is known about the factors that influence clover rot resistance. In this paper we evaluated the variation in clover rot susceptibility among a diverse collection of 113 red clover accessions, with the aim of identifying more resistant accessions. Clover rot susceptibility was assessed with a high throughput bio-test on young plants. We found significant variation in clover rot susceptibility, within and among accessions. ‘Tedi’, ‘Maro’ and ‘No 292’ were the most resistant accessions. Fifteen diploid accessions were more susceptible than the average accession with the cultivar ‘Nemaro’ being the most susceptible. Clover rot susceptibility was not correlated with isoflavone levels from Mullaney et al. (Agronomy abstract. ASA, Madison, p 195, 2000). Cultivars were more resistant than landraces and wild accessions and tetraploid cultivars were more resistant than diploid cultivars. Besides the in-depth analysis for clover rot susceptibility, possible correlations with plant architecture and other diseases were investigated. Growth habit, branching, plant yield, flowering date and susceptibility to mildew, virus and rust diseases were investigated in a 3-year field trial. Unlike previously suggested, clover rot susceptibility was not correlated with branching or with plant yield over three years. On the other hand, late flowering accessions and accessions with erect growth habit were less susceptible to clover rot. Clover rot susceptibility was not correlated with susceptibility to rust disease (Uromyces trifolii) or viral diseases, but negatively with susceptibility to mildew (Erysiphe polygoni). Because no completely resistant accessions were found, the best way to improve clover rot resistance would be to select recurrently for resistant genotypes among diverse cultivars and landraces with lower susceptibility. Tetraploidisation of diploid populations with a higher resistance level can provide an additional level of protection.
Similar content being viewed by others
References
Barnett OW, Diachun S (1986) Virus diseases of clovers: etiology and crop losses. In: Edwardson JR, Christie RG (eds) Viruses infecting forage legumes. University of Florida publishing, Gainesville, pp 625–675
Boland GJ, Hall R (1994) Index of host plants of Sclerotinia sclerotiorum. Can J of Plant Pathol 16:93–108
Boller B, Posselt UK, Veronesi F (2010) Handbook of plant breeding. Springer, Dordrecht
Dabkeviènë G, Dabkevièius Z (2005) Evaluation of wild red clover (Trifolium pratense L.) ecotypes and hybrid populations (Trifolium pratense L. × Trifolium diffusum Ehrh.) for clover rot resistance (Sclerotinia trifoliorum Erikks.). Biolojia 3:54–58
De Rijke E, Zafra-Gómez A, Ariese F, Brinkman UAT, Gooijer C (2001) Determination of isoflavone glucoside malonates in Trifolium pratense L. (red clover) extracts: quantification and stability studies. J Chromatogr A 932:55–64
Debnam JR, Smith IM (1976) Changes in the isoflavones and pterocarpans of red clover on infection with Sclerotinia trifoliorum and Botrytis cinerea. Physiol Plant P 9:9–23
Delclos B, Duc G (1996) Etude de la résistance à Sclerotinia trifoliorum chez le trèfle violet (Trifolium pratense L.). Dissertation, University of Paris
Dewitte A (2010) Exploitation of 2n pollen to create genetic variation in the genus Begonia. Dissertation, Universiteit Gent
Gururani MA, Venkatesh J, Upadhyaya CP, Nookaraju A, Pandey SK, Park SW (2012) Plant disease resistance genes: current status and future directions. Physiol Mol Plant P 78:51–65
Halimi ES, Rowe DE, Pratt RG (1998) Responses of alfalfa to stem-tip inoculations with five isolates of Sclerotinia trifoliorum. Crop Sci 38:1179–1182
He X, Lin L, Lian L (1996) Analysis of flavonoids from red clover by liquid chromatography-electrospray mass spectrometry. J Chromatogr A 755:127–132
King KC, Seppälä O, Neiman M (2012) Is more better? Polyploidy and parasite resistance. Biol Lett 8(4):598–600
Kohn LM (1979) Delimitation of the economically important plant pathogenic Sclerotinia species. Phytopathology 69:881–886
Kou Y, Wang S (2010) Broad-spectrum and durability: understanding of quantitative disease resistance. Curr Opin Plant Biol 13:1–5
Kouame CN, Quesenberry KH (1993) Cluster analysis of a world collection of red clover germplasm. Genet Resour Crop Ev 40:39–47
Macfoy CA, Smith IM (1979) Phytoalexin production and degradation in relation to resistance of clover leaves to Sclerotinia and Botrytis spp. Physiol Plant Pathol 14:99–111
Marum P, Smith RR, Grau CR (1994) Development of procedures to identify red clover resistant to Sclerotinia trifoliorum. Euphytica 77:257–261
Mullaney JM, Quesenberry KH, Macdonald GE (2000) Isoflavone concentration in the red clover core collection: part II. In Agronomy Abstracts. ASA, Madison, p 195
Öhberg H (2008) Studies of the persistence of red clover cultivars in Sweden with particular reference to Sclerotinia trifoliorum. Dissertation, Swedish University of Agricultural Studies
Öhberg H, Ruth P, Bang U (2005) Effect of ploidy and flowering type of red clover cultivars and of isolate origin on severity of clover rot, Sclerotinia trifoliorum. Phytopathology 153:505–511
Poland JA, Balint-Kurti JB, Wisser RJ, Pratt RC, Nelson RJ (2009) Shades of grey: the world of quantitative disease resistance. Trends Plant Sci 14:21–29
Pratt RG, Rowe DE (1995) Comparative pathogenicity of isolates of Sclerotinia trifoliorum and Sclerotinia sclerotiorum on alfalfa cultivars. Plant Dis 79:474–477
Saharan GS, Mehta N (2008) Sclerotinia diseases of crop plants: biology, ecology and disease management. Springer, New Delhi
Saint Clair DA (2010) Quantitative disease resistance and quantitative resistance loci in breeding. Annu Rev Phytopathol 48:247–268
Slusarenko AJ, Fraser RSS, van Loon LC (2000) Mechanisms of resistance to plant diseases. Kluwer Academic Publishers, Dordrecht
Taylor NL (2008) A century of clover breeding developments in the United States. Crop Sci 48:1–13
Taylor NL, Quesenberry KH (1996) Red clover science. Kluwer academic publishers, Dordrecht
Thordal-Christensen H (2003) Fresh insights into the processes of nonhost resistance. Curr Opin Plant Biol 6:351–357
Vaverka M, Vaverka S, Vichova J (2003) Resistance of cultivars of the Czech assortment of red clover Trifolium pratense L. to the stem and crown rot Sclerotinia trifoliorum Erikss. Czech J Genet Plant 39:326–329
Veitch NC (2009) Isoflavonoids of the Leguminosae. Nat Prod Rep 26:776–802
Vleugels T, De Riek J, Heungens K, van Bockstaele E, Baert J (2012) Analysis of genetic diversity in Sclerotinia populations from European red clover crops. J Plant Pathol 94:493–503
Vleugels T, Baert J, van Bockstaele E (2013) Morphological and pathogenic characterization of genetically diverse Sclerotinia isolates from European red clover crops (Trifolium Pratense L.). J Phytopathol 161:254–262
Ylimäki A (1967) Root rot as a cause of red clover decline in leys in Finland. Annales agriculturae Fenniae 6:1–59
Yli-Mattila T, Kalko G, Hannukkala A, Paavanen S, Hakala K (2009) Prevalence, species composition, genetic variation and pathogenicity of clover rot (Sclerotinia trifoliorum) and Fusarium spp. in red clover in Finland. Eur J Plant Pathol 126:13–27
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vleugels, T., Cnops, G. & van Bockstaele, E. Screening for resistance to clover rot (Sclerotinia spp.) among a diverse collection of red clover populations (Trifolium pratense L.). Euphytica 194, 371–382 (2013). https://doi.org/10.1007/s10681-013-0949-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-013-0949-4