Abstract
Ismailia Canal is one of the significant streams of the Nile River in Egypt. The study aimed to determine the water quality of Ismailia Canal based on the regional and seasonal variability of physicochemical parameters, irrigation criteria, and the irrigation water quality index (IWQI). It was observed that the physicochemical parameters were within the acceptable FAO irrigation limits. All cations and anions values were within the acceptable FAO limits for irrigation, except the potassium (K+) concentrations were over the permissible irrigation limits. The one-way analysis of variance (ANOVA) suggested a significant seasonal variation in the canal’s water quality concerning all parameters (p value ˂ 0.05). However, the regional variation among various sites was statistically insignificant (p value > 0.05). Statistical analysis was used to calculate the correlation coefficient between different parameters, and the study showed highly significant correlation coefficients between different pairs of water quality parameters. The correlation matrix showed that the pH significantly affected IWQI (r = 0.661). The irrigation criterion values for Ismailia Canal were good, and the WQI levels for irrigation utilization at all studied sites were satisfactory. Deterioration of water quality may occur due to industrial, municipal, and agricultural activities. Drainage water should be treated before being mixed with irrigation water to improve its suitability for irrigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Nile River is Egypt’s main supply of fresh water. Recently, Egypt has been experiencing water scarcity caused by various factors. The most well-known factors are ineffective irrigation systems and water waste. Egypt’s water consumption is rising due to rising population, rapid economic growth, and environmental destruction. The quality and scope of human-related activities in a region's basins substantially impact surface water quality. Wastewater discharges that have not been properly regulated have an immediate and long-term impact on consumers’ health (Goher et al., 2014). Because of the high concentration of organic pollutants that are hazardous to the aquatic environment and affect the health of the flora and fauna, the discharge of effluent without sufficient treatment greatly impacts the ecosystem (Oyekanmi et al., 2021).
In 1862, the construction of Ismailia Canal was completed providing Egypt with vital irrigation and drinking water (Goher et al., 2014). The canal begins at the Nile inlet in Cairo and continues east to the Ismailia governorate, passing through Cairo’s governorates. Port Said (90 km) and Suez (about 80 km) governorates are served by separate arms of the river, which separates near Ismailia town. The total area served by the river is approximately 108,200 fedden (Geriesh et al., 2008). It is used for irrigation, domestic, and industrial purposes, as well as a primary source of drinking water for many Egyptians, including those in the northern part of Great Cairo, Shubra El-Kheima, El-Amira, Mattaria, Musturod, Abu-Zaabal, Inchas, Belbeis, Abu-Hammad, Zagazig, and El-Tal el Kabeer.
Ismailia Canal is the furthest downstream from the main Nile River. Its water quality is susceptible to numerous sources of contamination. Shubra El-Kheima, Musturod, and Abu Zaabal are the three main industrial zones in Egypt, located upstream of Cairo on the western side of Ismailia Canal. Alum (aluminum sulfate), Abu Zaabal Fertilizers company, and a detergent business are located in these locations. Wastewater treatment plants discharge high levels of aluminum, iron, and manganese wastewater into the surrounding environment, causing significant changes in pH and chemical properties. There is much pollution from nearby communities and septic tanks and agricultural effluents; seep into the canal. Contaminated water poses a serious health risk due to reusing drainage water and pumping filthy groundwater. Many organic pollutants are known to be harmful or carcinogenic. Surface and ground waters must be studied for changing component levels to reduce pollution and enhance water quality (El-Amier et al., 2021).
It is important to consider the irrigation water’s salinity and ion toxicity before using it. When there is too much salt in the soil (sodicity), the soil structure breaks down. Waterways must be regularly monitored to detect any shifts in salt concentration. Plant growth can be affected by high levels of HCO3−, Cl−, Na+, Mg++, and other trace elements (Shrestha & Kazama, 2007). Heavy metals are regarded as dangerous and toxic when present in water bodies. Heavy metal contamination in wastewater can cause diseases like cancer, skin mutations, and mental disorders when it is deposited and consumed. To promote a healthy ecosystem, it is crucial to remove these metals from wastewater before releasing them into the environment (Adeleke et al., 2017).
The water quality index (WQI) is one of the best standard methods to assess a certain area’s overall water quality based on several different criteria (Kothari et al., 2021). To achieve the final score of WQI, many factors related to water quality are taken into account. It is one of the most effective ways of disseminating information regarding water quality (Cabassud et al., 2001). With the help of WQI, the general public, politicians, and other judges can quickly and easily learn about water quality in their area of interest (Rothmaier et al., 1997). Water resources in a community can be managed and exploited in various ways depending on the quality of the water (Kothari et al., 2021). Consequently, this research aims to investigate Ismailia Canal water quality for irrigation based on the regional and seasonal variability of physicochemical parameters, irrigation criteria, and the irrigation water quality index (IWQI).
Materials and methods
Description of the study area
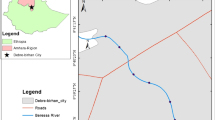
Ismailia Canal is one of the most important irrigation canals in Egypt. It was constructed to transport fresh water from River Nile in north Cairo (El-Mezalat) to Ismailia, Port Said, and Suez governorates (Fig. 1). It is 128 km long, 2.1 m in depth, and 18 m in width. The water canal’s flow rate is 433.56 m3/s. About 108.200 fedden are covered by the canal, which provides water for drinking and agriculture and is used in industrial processes (Goher et al., 2014).
Collection of water samples
The research samples were collected in polyethylene bottles from ten sites along Ismailia Canal and transferred to the laboratory in an ice box within 6 h of collection. Each location had three replicates taken in the same line: right, center, and left canal side. The water samples were collected in 2020 during the research period (winter, spring, summer, and autumn). Table 1 and Fig. 1 depict the locations of the sites.
Water quality characterization
Field measurements
At the sampling site, pH, electrical conductivity (EC), and dissolved oxygen (DO) were measured using pH meter (ORION) model 420A and electrical conductivity meter (WTW) (Inolab) level 1, respectively.
Laboratory analysis
The physicochemical parameters (total dissolved solids (TDS), cations (calcium (Ca++), magnesium (Mg++), sodium (Na+), and potassium (K+)), anions (chloride (Cl−), nitrate (NO3−), sulfate (SO4−−), bicarbonate (HCO3−), and carbonate (CO3)), heavy metals (Al+++, Cu++, Pb++, Zn++, Cr++, and Fe++), and biological parameters (biological oxygen demand (BOD), fecal coliform (FC), and E. coli) were analyzed in the laboratory using American Public Health Association (APHA) standard procedures (Grant & Greene, 2012). Dissolved oxygen was measured using dissolved oxygen meter (WTW) model OXI 315i and oxygen sensor (WTW) model DuroX325. The COD was measured using COD spectrophotometer (TURNER) model 690 with a COD reactor (HACH). The BOD was measured using BOD manometer (WTW) (Oxitop system) 12-bottle set. The anions were measured by ion chromatography (IC), model DX-500 chromatography system. In addition, the cations and heavy metals were measured by ICP-OES instrument (Inductively Coupled Argon Plasma-Optical Emission Spectroscopy) (Perkin Elmer Optima 3000 Redial, USA). All bacterial parameters were examined by using the membrane filter technique.
Irrigation water quality criteria
The presence of undesired elements determines the water’s appropriateness for irrigation. To assess the quality and irrigation suitability of these waters, the most frequently calculated irrigation criteria have been used. The following formulas were used to calculate the sodium adsorption ratio (SAR), residual sodium carbonate (RSC), sodium percentage (Na%), permeability index (PI%), magnesium hazard percentage (MH%), and Kelly’s index (KI), and their categories are described in Table 2.
Sodium adsorption ratio (SAR) = Na+ / √((Ca++ + Mg++) / 2) (Richards, 1954)
Residual sodium carbonate (RSC) mmole L−1 = [CO3−− + HCO3−]—[Ca++ + Mg++] (Murtaza et al., 2021)
Sodium percentage (Na%) = (Na+ + K+) / (Na+ + Ca++ + Mg++ + K+) × 100 (Oster & Sposito, 1980)
Permeability index (PI%) = (Na+ + √HCO3−) / (Ca++ + Mg++ + Na+) × 100 (Eyankware et al., 2018)
Magnesium hazard percentage (MH%) = Mg++ / (Ca++ + Mg++) × 100 (Zhang et al., 2021)
Kelly’s index (KI) = Na+ / (Ca++ + Mg++) (Shil et al., 2019)
Irrigation water quality index (IWQI)
The weighted arithmetic index method (Brown et al., 1972) has been used to calculate the WQI. Many scientists have relied on this mathematical method (Balan et al., 2012; Tyagi et al., 2013). The steps to arrive at a single WQI score included identifying the parameters, classifying them, assigning a relative weight to each, and then merging all the results (Şener et al., 2017; Taloor et al., 2020). Table 11 shows the factors utilized in irrigation water quality that were the most important in the selection process. The following equation was used to determine the parameter (Si) scores:
where Vactual is the monitored value of the n parameter and Videal is the ideal value for the parameter in pure water. Dissolved oxygen is 14.6 mgL−1, pH is 7, and all other values are identical to 0 (Boah et al., 2015). The Food and Agriculture Organization (FAO) (Lupien, 1994) has established a standard acceptable value for Standard. Furthermore, the following equation was used to determine the relative weight (Rwi).
where Wi was the weight of each parameter. Each parameter was assigned a weight according to the following equation:
Finally, the mathematical formula of the IWQI was given by the following equation:
Statistical analysis
The mean values, standard deviations, and the range of results were performed using Excel-Stat software. The Statistics Package for Social Science (SPSS software Version 20) was used to examine water quality data. In addition, the water quality data were tested for normality with the Shapiro–Wilk test. The relationship between the water quality indices was determined using the two-tail Spearman rank correlation. A correlation coefficient (r) spans from − 1 to + 1, a numerical value. Generally, the correlation between the parameters is strong when it is between 0.8 and 1 and moderate when it is between 0.5 and 0.8. One-way ANOVA was used to investigate the significant spatial and seasonal difference at a probability level of 0.05 (Faruque et al., 2005). Box plots were performed using SPSS to show a significant seasonal difference as per ANOVA.
Results and discussion
Water quality characterization
The water quality of Ismailia Canal determined many essential physicochemical parameters at ten different sites during various seasons, as shown in Tables 3, 4, and 5. In addition, ANOVA values of spatial and seasonal variation of water quality parameters were given in Tables 6 and 7, respectively. Box plots for the parameters showed seasonal and spatial statistically significant differences as per ANOVA, as represented in Fig. 2. The pH is an important metric that indicates the acceptability of water for various applications, and it is one of the physicochemical parameters chosen for water quality. The pH levels in this study ranged from 7.18 to 8.09 demonstrated the water’s alkaline nature. The slight increase in the pH at some sites may be due to the inputs from industrial wastewater. The pH alterations are connected to the changes in conductivity and bicarbonate content (Roy & Rhim, 2021). It has been found that pH did not vary significantly among sites (p value = 0.949) (Table 6). However, pH varied significantly between seasons (p value = 0.00) (Table 7). It is possible to use the electrical conductivity (EC) value to indicate the total amount of dissolved salts in water. The studied sites met the FAO’s irrigation water EC standards of 3,000 μS cm−1 based on their EC readings. Ben-Gal et al. (2008) obtained similar observations in their studies on different water bodies (Ben-Gal et al., 2008). The variation of EC between ten sites was not statistically significant (p value = 0.999); however, there was statistically significant variation among various seasons (p value = 0.00). As shown in Table 8, the EC had a significant negative correlation with E. coli and a significant positive correlation with DO, TDS, BOD, Ca++, Na+, and K+.
The distribution of flora and fauna is regulated by dissolved oxygen concentration (DO). According to the current study, dissolved oxygen concentration varied between 5.27 and 7.09 mg L−1. Seasonal variation had significant difference (p value = 0.00). This finding was in agreement with Ben-Gal et al. (2008). The DO values decreased in summer due to high temperature and the high decomposition rate of organic matters (Fig. 2). The DO had a significant negative correlation with E. coli and Mg++ and a positive correlation with TDS, Ca++, Na+, K+, and SO4−−.
It is widely accepted that total dissolved solids (TDS) are an essential metric for assessing water quality since it is directly associated with and affected by increased turbidity, hardness, alkalinity, and conductivity. The TDS showed differences ranging from 243.25 to 320.25 mg L−1. The TDS was found to vary significantly between studied seasons (p value = 0.00). Moreover, the TDS had a significant negative correlation with E. coli and a positive correlation with Ca++, Na+, K+, and SO4−−.
The biochemical oxygen demand (BOD) is a metric for determining the amount of organic matter in water bodies. Many studies investigated that contaminated water has increased BOD levels. In the present study, spatial variation in BOD did not exist (p value = 0.227). Seasonal variation showed statistical significant (p value = 0.00). The BOD concentration ranged from 3.75 to 9.75 mg L−1 indicating eutrophication of the water body. The BOD had a negative correlation with Cl− and SO4−−, besides a significant positive correlation with E. coli.
Potential human health problems are indicated by the presence of bacteria in surface water (Haque et al., 2019). The bacterial pollution of Ismailia Canal’s surface water was investigated during the study period by detecting the fecal coliform and E. coli levels (Table 3). The elevated coliform bacteria counts were attributed to the research area’s rapid population growth, which was facilitated by the discharge of domestic trash containing feces into city sewers. Furthermore, temperature and seasonal variations are important determinants of bacterial growth (White et al., 1991). As the temperature rises in the summer, the fecal coliform concentration also rises, due to many factors, such as the lack of water and the high organic matter concentration. Restricted water, high organic matter, and growth-supporting nutrient concentrations are necessary for a healthy ecosystem (Haque et al., 2019). The cold winter climatic conditions did not promote bacterial growth, resulting in a lower coliform count throughout the season (Tiefenthaler et al., 2008). The elevated counts could be attributed to high nutrient concentrations in the companies’ discharges. The ANOVA results were reported that there was a statistically significant difference between seasons concerning FC (p value = 0.00) and E. coli (p value = 0.00) (Table 7). The fecal coliform had a significant inverse relationship with NO3− and a positive relationship with E. coli. Additionally, E. coli had a significant negative correlation with Cl− and SO4−−.
Major ion concentrations control the basic hydrochemical properties of surface water (Zhang et al., 2019). All the Ca++, Mg++, and Na+ ions values were within the acceptable FAO limits for irrigation (Table 4). Due to salt concentration, a high Na+ content in irrigation water can be detrimental to crop production. All potassium (K+) concentrations measured at the study sites were over the FAO permitted level (2 mg L−1), posing a risk to vegetable production. Furthermore, the Ca++ ion concentration positively correlated with Na and K+ ions concentrations. The Na+ positively correlated with K+.
On the other hand, high Cl− ions impact plant growth by increasing osmotic pressure, inhibiting crop growth, and decreasing plant water availability. The excess absorbed Cl− ions in plant tissues accumulate in leaves, causing leaf burns, whereas using an excessive amount of NO3− decreases the crop yield and quality. The ion concentrations (Cl−, NO3−, SO4−−, HCO3−, and CO3−) analysis indicated the allowed limits for irrigation. The Cl− concentration was positively correlated with SO4−−. The highest averages of Cl− and NO3− concentrations (56.84 and 1.37 mg L−1, respectively) were recorded at site 6 (Discharging point of Misr Petroleum Company). High values of Cl− and NO3− at this site may be due to the mix of industrial effluent with Ismailia Canal water. ANOVA results suggested a statistically significant seasonal variation concerning cations and anions (Table 7). However, spatial variation in the cations and anions was found to be statistically insignificant (Table 6).
Heavy metal contamination of irrigation water has been recognized as a major environmental threat due to its non-biodegradable nature and extended biological half-life, as well as the potential accumulation in numerous body parts. Residents who consume crops and/or vegetables grown in contaminated areas are exposed to excessive heavy metals due to their deposition in agricultural soils caused by irrigation wastewater (Hussain et al., 2019). In the current study, the heavy metals concentrations in the studied location were within the acceptable FAQ irrigation limits. On the other hand, the seasonal variation was statistically significant (p value ˂ 0.05) (Table 7 and Fig. 2).
Irrigation water quality criteria
Sodium adsorption ratio (SAR)
High-sodium irrigation water is particularly problematic because of the negative consequences on the soil. A classification of salt hazards has also been included in the SAR, which may hinder the ability of plants to absorb water (Behboudi et al., 2018). Soil particles absorb Na+ and become associated with it. When the soil dries out, it hardens and compacts, making it more water-resistant. For soils with high SARs, certain fertilizers may be necessary. If appropriate concentrations of Ca++ and Mg++ ions are present in the soil, they will balance the effects of Na+ ions and aid in protecting healthy soil qualities (De las Heras & Mañas, 2020). In addition, surface water can be classified as excellent or good (10 to 18), dubious (18 to 26), or undesirable (more than 26) by utilizing SAR, which measures the quality of the water. The samples had a SAR ranging from 5.35 to 5.93 (Table 9). Accordingly, Ismailia Canal sampling sites results were excellent (Table 10). A plot of sample data on the US salinity diagram (Richards, 1954), in which the EC is taken as a salinity hazard and the SAR is taken as an alkalinity hazard (Fig. 3), revealed that all of the samples fell into the medium salinity-low sodium category of water and that they can be used for irrigation on all types of soil with little risk of harmful levels of exchangeable sodium.
USSL diagram for classifying irrigation waters based on SAR and EC as described by Richards (1954)
Residual sodium carbonate (RSC)
RSC is a valuable index for determining the adequacy of irrigation water due to its ability to examine the link between the quantity of carbonate (CO3−−) and bicarbonate (HCO3−) and the total Ca++ and Mg++. Waters containing high concentrations of HCO3− tend to precipitate Ca++ and Mg++ ions when the soil water becomes more concentrated. Soils watered with highly RSC water content may become unproductive due to sodium carbonate accumulation (Khalid, 2019). According to the current results, the RSC values were good for all selected sites (Tables 9 and 10).
Sodium percentage (Na%)
The danger of Na+ poisoning from surface water can be estimated through calculated the percentage of Na+ ions soluble in water content. Na+ percentage is a common statistical method used to determine the appropriateness of natural waters for irrigation due to the interaction of Na+ ions in the soil and limit its permeability (Shil et al., 2019). More than 60% of water’s sodium content can result in Na+ accumulations, leading to soil degradation. Soils with high levels of Na+ and CO3−− are alkaline, while those with high levels of Na+ and Cl− are saline soils (Salifu et al., 2017). Tables 9 and 10 show that all sites along Ismailia Canal exhibited safe Na% levels ranging from 37.99 to 40.12% during the study period. The Wilcox diagram (Wilcox, 1955) relating sodium percent and EC (Fig. 4) revealed that all of the samples were in the “Excellent to Good” range. As a result, the research area’s sites were suitable for irrigation.
Permeability index (PI%)
Irrigation efficiency can be measured using the permeability index (PI). The concentrations of Na+, Ca++, Mg++, and HCO3− ions are all considered by the PI. The PI value in the research area ranged from 48.06 to 51.20 (Table 9). All collected samples had a moderate PI which increased the irrigation possibility. PI may have increased due to the increased solubility of carbonate and cation exchange in minerals, such as calcite and dolomite (Panneerselvam et al., 2021).
Magnesium hazard percentage (MH%)
In most streams, Ca++ and Mg++ concentrations are in balance. The alkalinity is a phenomenon occurs through soil water intrusion is impeded and crop yields are reduced when Mg++ ions and clay particles are present in high concentrations (Omar et al., 2019). Water samples had MH ranging from 27.15 to 32.85%. All tested samples have less than 50% moisture content, making them suitable for irrigation.
Kelly’s index (KI)
In the current research, Kelly’s index is used to determine irrigation water quality. This value is derived from the water’s Na+, Ca++, and Mg++ concentrations. Water with a KI value higher than one should be avoided due to its high Na+ concentration. KI values in this study varied from 0.51 to 0.55 for a representative water sample (Table 9).
Irrigation water quality index (IWQI)
Ismailia Canal water quality index (WQI) was computed using the 10 physicochemical criteria and the weighted arithmetic index approach. Tables 11 and 12 show the parameter scores (Si) as defined by FAO (Lupien, 1994), the ideal value (Videal), weight (Wi), relative weight (RWi), and IWQI. According to Table 2, the IWQI values varied from 6.84 to 16.7, indicating that the water of the analyzed sample sites is appropriate for irrigation. The discharges of the Misr Petroleum and Abu Zaabal fertilizer companies might affect the WQI values at sites 6 and 9, respectively. The IWQI had a significant positive correlation with the pH (r = 0.661) (Table 8). The obtained data also revealed that the drainage water of companies and cities must be thoroughly treated before discharging in the canal to avoid pollution of the canal stream.
As shown in Table 13, the SAR had a high negative correlation with MH% (r = − 0.939), substantial positive correlations with Na% (r = 0.951) and KI (r = 0.93). In contrast, there was a substantial negative correlation between the Na% and the MH% (r = − 0.988) and a strong positive association between the Na% and the KI (r = 0.924). The PI% had a statistically significant negative association with MH% (r = − 0.636). Also, there was a negative correlation between MH% and KI (r = − 0.924).
Conclusions
According to the classification of irrigation water quality index (IWQI), Ismailia Canal water is acceptable for irrigation according to available data, based on the findings of physicochemical analyses and irrigation criteria. The regional variance between the various research sites was statistically insignificant (p value > 0.05). On the other hand, seasonal variation was statistically significant (p value 0.05). Therefore, this study recommended conducting studies on the targeted area to shade more light on this important area on a regular and continuous basis in future works to avoid pollution, monitor any change in water quality, determine the effects of pollution on surface water, and determine its suitability for irrigation and human uses. In addition, irrigation water, industrial, municipal, and agricultural drainages must be appropriately treated before blending with canal water.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adeleke, A. R. O., Abdul Latiff, A. A., Daud, Z., Mat Daud, N. F., & Aliyu, M. K. (2017). Heavy metal removal from wastewater of palm oil mill using developed activated carbon from coconut shell and cow bones. Key Engineering Materials.
Balan, I. N., Shivakumar, M., & Kumar, P. (2012). An assessment of groundwater quality using water quality index in Chennai, Tamil Nadu, India. Chronicles of Young Scientists, 3(2).
Behboudi, F., Tahmasebi Sarvestani, Z., Kassaee, M. Z., Modares Sanavi, S., & Sorooshzadeh, A. (2018). Improving growth and yield of wheat under drought stress via application of SiO2 nanoparticles. Journal of Agricultural Science and Technology, 20(7), 1479–1492.
Ben-Gal, A., Ityel, E., Dudley, L., Cohen, S., Yermiyahu, U., Presnov, E., Zigmond, L., & Shani, U. (2008). Effect of irrigation water salinity on transpiration and on leaching requirements: A case study for bell peppers. Agricultural Water Management, 95(5), 587–597.
Boah, D. K., Twum, S. B., & Pelig-Ba, K. B. (2015). Mathematical computation of water quality index of Vea dam in upper east region of Ghana. Environmental Sciences, 3(1), 11–16.
Brown, R. M., McClelland, N. I., Deininger, R. A., & O’Connor, M. F. (1972). A water quality index—crashing the psychological barrier. In Indicators of environmental quality (pp. 173–182). Springer.
Cabassud, C., Burgaud, C., & Espenan, J.-M. (2001). Spring water treatment with ultrafiltration and stripping. Desalination, 137(1–3), 123–131.
Das, S., & Nag, S. (2015). Deciphering groundwater quality for irrigation and domestic purposes–a case study in Suri I and II blocks, Birbhum District, West Bengal. India. Journal of Earth System Science, 124(5), 965–992.
De las Heras, J., & Mañas, P. (2020). Reclaimed wastewater to irrigate olive groves and vineyards: Effects on soil properties. Agronomy, 10(5), 649.
El-Amier, Y. A., Kotb, W. K., Bonanomi, G., Fakhry, H., Marraiki, N. A., & Abd-ElGawad, A. M. (2021). Hydrochemical assessment of the irrigation water quality of the El-Salam canal, Egypt. Water, 13(17), 2428.
Eyankware, M., Nnajieze, V., & Aleke, C. (2018). Geochemical assessment of water quality for irrigation in abandoned limestone quarry pit at Nkalagu area, southern Benue Trough, Nigeria. Environmental Earth Sciences, 77(3), 1–12.
Faruque, S. M., Naser, I. B., Islam, M. J., Faruque, A., Ghosh, A., Nair, G. B., Sack, D. A., & Mekalanos, J. J. (2005). Seasonal epidemics of cholera inversely correlate with the prevalence of environmental cholera phages. Proceedings of the National Academy of Sciences, 102(5), 1702–1707.
Geriesh, M. H., Balke, K.-D., & El-Rayes, A. E. (2008). Problems of drinking water treatment along Ismailia Canal Province, Egypt. Journal of Zhejiang University Science B, 9(3), 232–242.
Goher, M. E., Hassan, A. M., Abdel-Moniem, I. A., Fahmy, A. H., & El-sayed, S. M. (2014). Evaluation of surface water quality and heavy metal indices of Ismailia Canal, Nile River, Egypt. The Egyptian Journal of Aquatic Research, 40(3), 225–233.
Grant, R., & Greene, D. (2012). The health care home model: Primary health care meeting public health goals. American Journal of Public Health, 102(6), 1096–1103.
Haque, M. A., Jewel, M. A. S., & Sultana, M. P. (2019). Assessment of physicochemical and bacteriological parameters in surface water of Padma River, Bangladesh. Applied Water Science, 9(1), 1–8.
Hussain, S., Habib-Ur-Rehman, M., Khanam, T., Sheer, A., Kebin, Z., & Jianjun, Y. (2019). Health risk assessment of different heavy metals dissolved in drinking water. International Journal of Environmental Research and Public Health, 16(10), 1737.
Khalid, S. (2019). An assessment of groundwater quality for irrigation and drinking purposes around brick kilns in three districts of Balochistan province, Pakistan, through water quality index and multivariate statistical approaches. Journal of Geochemical Exploration, 197, 14–26.
Kothari, V., Vij, S., Sharma, S., & Gupta, N. (2021). Correlation of various water quality parameters and water quality index of districts of Uttarakhand. Environmental and Sustainability Indicators, 9, 100093.
Lupien, J. R. (1994). Dietary assessment issues of concern to policymakers: Statement from the Food and Agriculture Organization of the United Nations. The American Journal of Clinical Nutrition, 59(1), 269S-270S.
Murtaza, G., Rehman, M., Qadir, M., Shehzad, M., Zeeshan, N., Ahmad, H., Farooqi, Z., & Naidu, R. (2021). High residual sodium carbonate water in the Indian subcontinent: Concerns, challenges and remediation. International Journal of Environmental Science and Technology, 18(10), 3257–3272.
Omar, H., Aboella, W. A., Hassan, M. M., Hassan, A., Hassan, P., Elshall, A., Khaled, D., Mostafa, M., Tawadros, P. Z., & Hossam Eldin, M. (2019). Comparative study between intrathecal dexmedetomidine and intrathecal magnesium sulfate for the prevention of post-spinal anaesthesia shivering in uroscopic surgery;(RCT). BMC Anesthesiology, 19(1), 1–10.
Oster, J., & Sposito, G. (1980). The Gapon coefficient and the exchangeable sodium percentage-sodium adsorption ratio relation. Soil Science Society of America Journal, 44(2), 258–260.
Oyekanmi, A., Ahmad, A., Mohd Setapar, S. H., Alshammari, M. B., Jawaid, M., Hanafiah, M. M., Abdul Khalil, H., & Vaseashta, A. (2021). Sustainable durio zibethinus-derived biosorbents for congo red removal from aqueous solution: Statistical optimization, isotherms and mechanism studies. Sustainability, 13(23), 13264.
Panneerselvam, B., Muniraj, K., Thomas, M., Ravichandran, N., & Bidorn, B. (2021). Identifying influencing groundwater parameter on human health associate with irrigation indices using the automatic linear model (ALM) in a semi-arid region in India. Environmental Research, 202, 111778.
Ravikumar, P., Somashekar, R., & Angami, M. (2011). Hydrochemistry and evaluation of groundwater suitability for irrigation and drinking purposes in the Markandeya River basin, Belgaum District, Karnataka State. India. Environmental Monitoring and Assessment, 173(1), 459–487.
Richards, L. A. (1954). Diagnosis and improvement of saline and alkali soils (vol. 78). LWW.
Rothmaier, R., Weidenmann, A., & Botzenhart, K. (1997). Transport of Escherichia coli through soil to groundwater traced by randomly amplified polymorphic DNA (RAPD). Water Science and Technology, 35(11–12), 351–357.
Roy, S., & Rhim, J.-W. (2021). Anthocyanin food colorant and its application in pH-responsive color change indicator films. Critical Reviews in Food Science and Nutrition, 61(14), 2297–2325.
Salifu, M., Aidoo, F., Hayford, M. S., Adomako, D., & Asare, E. (2017). Evaluating the suitability of groundwater for irrigational purposes in some selected districts of the Upper West region of Ghana. Applied Water Science, 7(2), 653–662.
Şener, Ş., Şener, E., & Davraz, A. (2017). Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Science of the Total Environment, 584, 131–144.
Shah, B., Kansara, B., Shankar, J., Soni, M., Bhimjiyani, P., Bhanushali, T., Shah, M., & Sircar, A. (2019). Reckoning of water quality for irrigation and drinking purposes in the konkan geothermal provinces, Maharashtra. India. Groundwater for Sustainable Development, 9, 100247.
Shil, S., Singh, U. K., & Mehta, P. (2019). Water quality assessment of a tropical river using water quality index (WQI), multivariate statistical techniques and GIS. Applied Water Science, 9(7), 1–21.
Shrestha, S., & Kazama, F. (2007). Assessment of surface water quality using multivariate statistical techniques: A case study of the Fuji river basin, Japan. Environmental Modelling & Software, 22(4), 464–475.
Taloor, A. K., Pir, R. A., Adimalla, N., Ali, S., Manhas, D. S., Roy, S., & Singh, A. K. (2020). Spring water quality and discharge assessment in the Basantar watershed of Jammu Himalaya using geographic information system (GIS) and water quality Index (WQI). Groundwater for Sustainable Development, 10, 100364.
Tiefenthaler, L. L., Stein, E. D., & Schiff, K. C. (2008). Watershed and land use–based sources of trace metals in urban storm water. Environmental Toxicology and Chemistry: An International Journal, 27(2), 277–287.
Tyagi, S., Sharma, B., Singh, P., & Dobhal, R. (2013). Water quality assessment in terms of water quality index. American Journal of Water Resources, 1(3), 34–38.
White, P. A., Kalff, J., Rasmussen, J. B., & Gasol, J. M. (1991). The effect of temperature and algal biomass on bacterial production and specific growth rate in freshwater and marine habitats. Microbial Ecology, 21(1), 99–118.
Wilcox, L. (1955). Classification and use of irrigation waters. US Department of Agriculture.
Zhang, Q., Qian, H., Xu, P., Hou, K., & Yang, F. (2021). Groundwater quality assessment using a new integrated-weight water quality index (IWQI) and driver analysis in the Jiaokou Irrigation District, China. Ecotoxicology and Environmental Safety, 212, 111992.
Zhang, W., Ma, L., Abuduwaili, J., Ge, Y., Issanova, G., & Saparov, G. (2019). Hydrochemical characteristics and irrigation suitability of surface water in the Syr Darya River, Kazakhstan. Environmental Monitoring and Assessment, 191(9), 1–17.
Acknowledgements
The authors are grateful to the National Water Research Center and the Environmental Quality Monitoring staff at the Central Laboratory for their assistance and cooperation.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amer, A.S., Mohamed, W.S. Assessment of Ismailia Canal for irrigation purposes by water quality indices. Environ Monit Assess 194, 862 (2022). https://doi.org/10.1007/s10661-022-10350-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10350-y