Abstract
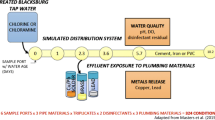
Variability in the concentration of lead and copper sampled at consumers’ taps poses challenges to assessing consumer health threats and the effectiveness of corrosion control. To examine the minimum variability that is practically achievable, standardized rigs with three lead and copper containing plumbing materials (leaded brass, copper tube with lead solder, and a lead copper connection) were deployed at five utilities and sampled with regimented protocols. Variability represented by relative standard deviation (RSD) in lead release was high in all cases. The brass had the lowest variability in lead release (RSD = 31 %) followed by copper-solder (RSD = 49 %) and lead-copper (RSD = 80 %). This high inherent variability is due to semi-random detachment of particulate lead to water, and represents a modern reality of water lead problems that should be explicitly acknowledged and considered in all aspects of exposure, public education, and monitoring.






Similar content being viewed by others
References
Al-Jasser, A. O. (2007). Chlorine decay in drinking-water transmission and distribution systems: pipe service age effect. Water Research, 41(2), 387–396.
American Water Works Association. (2002). Effects of water age on drinking water distribution system water quality. Washington, DC: U.S. Environmental Protection Agency.
Arnold, R. B., & Edwards, M. (2012). Potential reversal and the effects of flow pattern on galvanic corrosion of lead. Environmental Science & Technology, 46(20), 10941–10947. doi:10.1021/es3017396.
Arnold, R. B., Griffin, A., & Edwards, M. (2012). Controlling copper corrosion in new construction by organic matter removal. Journal American Water Works Association, 104. doi:10.5942/jawwa.2012.104.0072.
Boyd, G., Pierson, G., Kirmeyer, G., Britton, M., & English, R. (2008a). Lead release from new end-use plumbing components in Seattle public schools. Journal American Water Works Association, 100(3), 105–114.
Boyd, G., Pierson, G., Kirmeyer, G., & English, R. (2008b). Lead variability testing in Seattle public schools. Journal American Water Works Association, 100(2), 53–64.
Britton, A., & Richards, W. (1981). Factors influencing plumbosolvency in Scotland. Journal of Institution of Water Engineers and Scientists, 35(4), 349–364.
Broo, A. E., Berghult, B., & Hedberg, T. (1997). Copper corrosion in drinking water distribution systems—the influence of water quality. Corrosion Science, 39(6), 1119–1132.
Brown, R. A., & Cornwell, D. A. (2015). High velocity household and service line flushing following LSL replacement. Journal American Water Works Association. doi:10.5942/jawwa.2015.107.0012.
Calle, G. R., Vargas, I. T., Alsina, M. A., Pasten, P. A., & Pizarro, G. E. (2007). Enhanced copper release from pipes by alternating stagnation and flow events. Environmental Science & Technology, 41(21), 7430–7436.
Cantor, A. F., Park, J., & Vaiyavatjamai, P. (2003). Effect of chlorine on corrosion in drinking water systems. Journal American Water Works Association, 95(5), 112–123.
Cartier, C., Laroche, L., Deshommes, E., Nour, S., Richard, G., Edwards, M., & Prévost, M. (2011). Investigating dissolved lead at the tap using various sampling protocols. Journal American Water Works Association, 103(3), 55–67. 10.
Cartier, C., Arnold, R. B., Jr., Triantafyllidou, S., Prévost, M., & Edwards, M. (2012). Effect of flow rate and lead/copper pipe sequence on lead release from service lines. Water Research, 46(13), 4142–4152.
Clark, B., Masters, S., & Edwards, M. (2014). Profile sampling to characterize particulate lead risks in potable water. Environmental Science & Technology, 48(12), 6836–6843.
Clement, M., Seux, R., & Rabarot, S. (2000). A practical model for estimating total lead intake from drinking water. Water Research, 34(5), 1533–1542.
Colling, J. H., Croll, B. T., Whincup, P., & Harward, C. (1992). Plumbosolvency effects and control in hard waters. Water and Environment Journal, 6(4), 259–268.
Colling, J., Whincup, P., & Hayes, C. (1987). The measurement of plumbosolvency propensity to guide the control of lead in tapwaters. Water and Environment Journal, 1(3), 263–269.
Crozes, G. F., & Cushing, R. S. (2000). Evaluating biological regrowth in distribution systems. Denver: AWWA Research Foundation and American Water Works Association.
De Rosa, S., & Williams, S. (1992). Particulate lead in water supplies-final report to the Department of the Environment—May 1987 to December 1991. Wiltshire: Department of the Environment.
Del Toral, M. A., Porter, A., & Schock, M. R. (2013). Detection and evaluation of elevated lead release from service lines: a field study. Environmental Science & Technology, 47(16), 9300–9307.
Deshommes, E., Prevost, M., Levallois, P., Lemieux, F., & Nour, S. (2013). Application of lead monitoring results to predict 0–7 year old children's exposure at the tap. Water Research, 47(7), 2409–2420.
DiGiano, F. A., Zhang, W. D., & Travaglia, A. (2005). Calculation of the mean residence time in distribution systems from tracer studies and models. Journal of Water Supply: Research and Technology—Aqua, 54(1), 1–14.
Economic and Engineering Services. (1990). Lead control strategies. Denver: AWWARF.
Edwards, M. (2014). Fetal death and reduced birth rates associated with exposure to lead-contaminated drinking water. Environmental Science & Technology, 48(1), 739–746.
Edwards, M., & Dudi, A. (2004). Role of chlorine and chloramine in corrosion of lead-bearing plumbing materials. Journal American Water Works Association, 96(10), 69–81.
Edwards, M., & Triantafyllidou, S. (2007). Chloride-to-sulfate mass ratio and lead leaching to water. Journal American Water Works Association, 99(7), 96–109.
Edwards, M., Triantafyllidou, S., & Best, D. (2009). Elevated blood lead in young children due to leadcontaminated drinking water: Washington, DC, 2001–2004. Environmental Science & Technology, 43(5), 1618–1623.
Edwards, M., Jacobs, S., & Russell, T. (2000). The blue water phenomenon. Journal American Water Works Association, 92(7), 72–82.
Edwards, M. A., Powers, K., Hidmi, L., & Schock, M. R. (2001). The role of pipe ageing in copper corrosion by-product release. Water Science & Technology: Water Supply, 1(3), 25–32.
Ferguson, J. F., von Franque, O., & Schock, M. R. (1996). Corrosion of copper in potable water systems. Chapter 5 in internal corrosion of water distribution systems (2nd ed.). Denver: American Water Works Association Research Foundation.
Grace, S., Lytle, D. A., & Goltz, M. N. (2012). Control of new copper corrosion in high-alkalinity drinking water. Journal American Water Works Association, 104(1), 39–40.
Gregory, R., & Jackson, P.J. (1983). Reducing lead in drinking water. Report 219-S, Water Research Centre, Stevenage Laboratory.
Gronberg, R. (2007). Durham’s reassuring lead-testing results appear to have missed the glass. The Herald Sun.
Grumbles, B. (2004). Memorandum: lead and copper rule—clarification of requirements for collecting samples and calculating compliance. Washington, DC: U.S. Environmental Protection Agency.
HDR Engineering Inc. (2011). Study of lead corrosion in the city of Rochester Water Distribution System. Bellevue, WA.
Hill, C. P., & Cantor, A. F. (2011). Internal corrosion control in water distribution systems (1st ed.). Denver: American Water Works Association.
Imran, S., Dietz, J., Mutoti, G., Taylor, J., & Randall, A. (2005). Modified Larsons ratio incorporating temperature, water age, and electroneutrality effects on red water release. Journal of Environmental Engineering, 131(11), 1514–1520.
Johnson, J., Tran, T., & Yorton, R. (1993). Evaluation of corrosion control alternatives to meet the lead and copper rule for Eastern Massachusetts. Journal of New England Water Works Association, 107(1), 24–45.
Karalekas, P., Ryan, C., & Taylor, F. (1983). Control of lead, copper, and iron pipe corrosion in Boston. Journal American Water Works Association, 75(2), 92–95.
Kerneis, A., Nakache, F., Deguin, A., & Feinberg, M. (1995). The effects of water residence time on the biological quality in a distribution network. Water Research, 29(7), 1719–1727.
Kirmeyer, G., Murphy, B., & Sandvig, A. (2005). Post-optimization lead and copper control monitoring strategies (1st ed.). Denver: American Water Works Association Research Foundation.
Kuch, A., & Wagner, I. (1983). A mass transfer model to describe lead concentrations in drinking water. Water Research, 17(10), 1303–1307.
Lagos, G. E., Cuadrado, C. A., & Letelier, M. V. (2001). Aging of copper pipes by drinking water. Journal American Water Works Association, 93(11), 94–103.
Lee, R., Becker, W., & Collins, D. (1989). Lead at the tap: sources and control. Journal American Water Works Association, 81(7), 52–62.
Lehtola, M. J., Laxander, M., Miettinen, I. T., Hirvonen, A., Vartiainen, T., & Martikainen, P. J. (2006). The effects of changing water flow velocity on the formation of biofilms and water quality in pilot distribution system consisting of copper or polyethylene pipes. Water Research, 40(11), 2151–2160.
Leonnig, C. (2004). EPA concludes WASA broke lead law: order cites violations in six categories but levies no penalties. Washington Post, p. B01.
Levin, R., Brown, M. J., Kashtock, M. E., Jacobs, D. E., Whelan, E. A., & Rodman, J. (2008). Lead exposures in U.S. children, 2008: implications for prevention. Environmental Health Perspectives, 116(10), 1285–1293.
Lintereur, P., Duranceau, S., Taylor, J., & Stone, E. (2010). Sodium silicate impacts on lead release in a blended potable water distribution system. Desalination and Water Treatment, 16(1–3), 427–438.
Lu, C., Biswas, P., & Clark, R. (1995). Simultaneous transport of substrates, disinfectants and microorganisms. Water Research, 29(3), 881–894.
Marcus, A.H., Hogan, K., & Cox, M. (1990). Variability of household water lead levels in american cities. Report from Battelle Arlington Operations to U.S. EPA. Contract No. 68-D8-0115.
Masters, S., & Edwards, M. A. (2015). Increased lead in water associated with iron corrosion. Environmental Engineering Science, 32(5), 361–369.
Masters, S., Parks, J., Atassi, A., & Edwards, M. A. (2015a). Distribution system water age can create premise plumbing corrosion hotspots. Environmental Monitoring and Assessment. doi:10.1007/s10661-015-4747-4.
Masters, S., Wang, H., Pruden, A., & Edwards, M. A. (2015b). Redox gradients in distribution systems influence water quality, corrosion, and microbial ecology. Water Research, 68(1), 140–149.
Matthew, G. K. (1981). Lead in drinking water and health. Science of the Total Environment, 18, 61–75.
McNeill, L., & Edwards, M. (2004). Importance of Pb and Cu particulate species for corrosion control. Journal of Environmental Engineering, 130(2), 136–144.
Moore, M. (1973). Plumbosolvency of waters. Nature. doi:10.1038/243222a0.
Mutoti, G., Dietz, J. D., Arevalo, J., & Taylor, J. S. (2007). Combined chlorine dissipation: pipe material, water quality, and hydraulic effects. Journal American Water Works Association, 99(10), 96–106.
Parks, J., Edwards, M., & Atassi, A. (2014). Non-intrusive methodology for assessing lead and copper corrosion (no. 4317). Denver: Water Research Foundation.
Pocock, S. J. (1980). Factors influencing household water lead: a British national survey. Archives of Environmental Health, 35(1), 45–51.
Rajaratnam, G., Winder, C., & An, M. (2002). Metals in drinking water from new housing estates in the Sydney area. Environmental Research, 89(2), 165–170.
Reiber, S., Poulsom, S., Perry, S., Edwards, M., Patel, S., & Dodrill, D. (1997). A general framework for corrosion control based on utility experience. Denver: American Water Works Association.
Renner, R. (2006). Lead in water linked to coagulant. Environmental Science & Technology, 40(17), 5164–5165.
Rushing, J., & Edwards, M. (2004). The role of temperature gradients in residential copper pipe corrosion. Corrosion Science, 46(8), 1883–1894.
Sandvig, A., Kwan, P., Kirmeyer, G., Maynard, B., Mast, R., & Rhodes, R. T. (2008). Contribution of service line and plumbing fixtures to lead and copper rule compliance issues: Awwarf Report 91229 (Pap/Cdr edition.). Denver: Intl Water Assn.
Scardina, P., Edwards, M., Bosch, D., Loganathan, G. V., & Dwyer, S. (2008). Assessment of non-uniform corrosion in copper piping. Denver, CO: American Water Works Association Research Foundation.
Schaut, G. (1942). The action of a chlorinated water supply upon lead pipe. American Journal of Pharmacy, 114, 241–249.
Schock, M. R. (1990). Causes of temporal variability of lead in domestic plumbing systems. Environmental Monitoring and Assessment, 15(1), 59–82.
Schock, M., DeSantis, M., Metz, D., Welch, M., Hyland, R., & Nadagouda, M. et al. (2008). Revisiting the pH effect on orthophosphate control of plumbosolvency. Presented at American Water Works Association Annual Conference and Exposition, Atlanta, GA.
Schock, M. R., & Gardels, M. (1983). Plumbosolvency reduction by high pH and low carbonate—solubility relationships. Journal American Water Works Association, 75(2), 87–91.
Schock, M., & Sandvig, A. (2009). Long-term effects of orthophosphate treatment on copper concentration. Journal American Water Works Association, 101(7), 71–82.
Schock, M. R., Wagner, I., & Oliphant, R. (1996). Corrosion and solubility of lead in drinking water. Chapter 4 in internal corrosion of water distribution systems (2nd ed.). Denver: American Water Works Association Research Foundation.
Schock, M. R., Cantor, A. F., Triantafyllidou, S., DeSantis, M. K., & Scheckel, K. G. (2014). Importance of pipe deposits to lead and copper rule compliance. Journal American Water Works Association, 106(7), E336–E349.
Sheiham, I., & Jackson, P. (1981). Journal of the Institution of Water Engineers and Scientists, 135(6), 419–515.
Stith, P., & Raynor, D. (2006). Silence, flawed test data hide lead contamination. Raleigh: News and Observer.
Tang, Z., Hong, S., Xiao, W., & Taylor, J. (2006). Impacts of blending ground, surface, and saline waters on lead release in drinking water distribution systems. Water Research, 40(5), 943–950.
Triantafyllidou, S., & Edwards, M. (2009). Lead (Pb) in U.S. drinking water: school case studies, detection challenges and public health considerations. New Haven: Yale University.
Triantafyllidou, S., & Edwards, M. (2011). Galvanic corrosion after simulated small-scale partial lead service line replacements. Journal American Water Works Association, 103(9), 85–99.
Triantafyllidou, S., & Edwards, M. (2012). Lead (Pb) in tap water and in blood: implications for lead exposure in the United States. Critical Reviews in Environmental Science and Technology, 42(13), 1297–1352.
Triantafyllidou, S., Parks, J., & Edwards, M. (2007). Lead particles in potable water. Journal American Water Works Association, 99(6), 107–117,12.
Triantafyllidou, S., Nguyen, C. K., Zhang, Y., & Edwards, M. A. (2013). Lead (Pb) quantification in potable water samples: implications for regulatory compliance and assessment of human exposure. Environmental Monitoring and Assessment, 185(2), 1355–1365.
Triantafyllidou, S., Schock, M., DeSantis, M., & White, C. (2015). Low contribution of PbO2-coated lead service lines to water lead contamination at the tap. Environmental Science & Technology, 49(6), 3746–3754.
Troesken, W. (2006). The great lead water pipe disaster. Cambridge: The MIT Press.
Turek, N. F., Kasten, L., Lytle, D. A., & Goltz, M. N. (2011). Impact of plumbing age on copper levels in drinking water. Journal of Water Supply: Research and Technology—Aqua. doi:10.2166/aqua.2011.014.
U.S. Environmental Protection Agency. (1991). Safe drinking water act lead and copper rule (LCR). Federal Register, 56, 26460–26564.
U.S. Environmental Protection Agency. (2014). Lead and Copper Rule long-term revisions white paper. Washington DC: U.S. Environmental Protection Agency.
US EPA, O. (2005). Is there lead in my drinking water? http://water.epa.gov/drink/info/lead/leadfactsheet.cfm#two. Accessed 1 March 2015.
van den Hoven, T. (1985). How to determine and control lead levels in tap water: The Dutch approach. in proceedings of the 13th Annual AWWA Water Quality and Technology Conference. Denver: American Water Works Association.
Van Der Leer, D., Weatherill, N. P., Sharp, R. J., & Hayes, C. R. (2002). Modelling the diffusion of lead into drinking water. Applied Mathematical Modelling, 26(6), 681–699.
Vargas, I. T., Pavissich, J. P., Olivares, T. E., Jeria, G. A., Cienfuegos, R. A., Pasten, P. A., & Pizarro, G. E. (2010). Increase of the concentration of dissolved copper in drinking water systems due to flow-induced nanoparticle release from surface corrosion by-products. Corrosion Science, 52(10), 3492–3503.
Via, S. (2010). Public stakeholder meeting minutes lead and copper rule: proposed long-term regulatory revisions. Philadelphia: U.S. Environmental Protection Agency.
Wang, Z., Devine, H., Zhang, W., & Waldroup, K. (2014). Using a GIS and GIS-assisted water quality model to analyze the deterministic factors for lead and copper corrosion in drinking water distribution systems. Journal of Environmental Engineering, 140(9), A4014004.
Wysock, B. M., Sandvig, A. M., Schock, M. R., & Frebis, C. P. (1995). Statistical procedures for corrosion studies. Journal American Water Works Association, 87(7), 99–112.
Zhang, Y., Love, N., & Edwards, M. (2009). Nitrification in drinking water systems. Critical Reviews in Environmental Science and Technology, 39(3), 153–208.
Acknowledgments
The authors would like to thank the Water Research Foundation for their generous support of this study. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the writers and do not necessarily reflect the views of the Water Research Foundation. Navarre Bartz, Brent Casteele, Jacquelyn Dalrymple, Christopher Stamopolous, and Justin St. Clair were invaluable with the assistance they provided in designing and constructing the metal test pieces and the pipe rigs. We would also like to thank the personnel at the five participating utilities that helped install and monitor the pipe rigs.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1462 kb)
Rights and permissions
About this article
Cite this article
Masters, S., Parks, J., Atassi, A. et al. Inherent variability in lead and copper collected during standardized sampling. Environ Monit Assess 188, 177 (2016). https://doi.org/10.1007/s10661-016-5182-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5182-x