Abstract
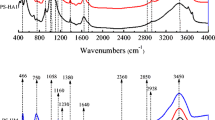
Soil organic matter (SOM) content is the major soil component affecting pesticide sorption. However, recent studies have highlighted the fact that it is not the total carbon content of the organic matter, but its chemical structure which have a profound effect on the pesticide’s sorption. In the present study, sorption of atrazine and metsulfuron-methyl herbicides was studied in four SOM fractions viz. commercial humic acid, commercial lignin, as well as humic acid and humin extracted from a compost. Sorption data was fitted to the Freundlich adsorption equation. In general, the Freundlich slope (1/n) values for both the herbicides were <1. Except for atrazine sorption on commercial humic acid, metsulfuron-methyl was more sorbed. Desorption results suggested that atrazine was more desorbed than metsulfuron-methyl. Lignin, which showed least sorption of both the herbicides, showed minimum desorption. Sorption of atrazine was best positively correlated with the alkyl carbon (adjusted R 2 = 0.748) and carbonyl carbon (adjusted R 2 = 0.498) but, their effect was statistically nonsignificant (P = 0.05). Metsulfuron-methyl sorption showed best positive correlation with carbonyl carbon (adjusted R 2 = 0.960; P = 0.05) content. Sorption of both the herbicides showed negative correlation with O/N-alkyl carbon. Correlation of herbicide’s sorption with alkyl and carbonyl carbon content of SOM fractions suggested their contribution towards herbicide sorption. But, sorption of metsulfuron-methyl, relatively more polar than atrazine, was mainly governed by the polar groups in SOM. IR spectra showed that H-bonds and charge-transfer bonds between SOM fraction and herbicides probably operated as mechanisms of adsorption.





Similar content being viewed by others
References
Ahangar, A. G. (2010). Does soil organic matter chemistry have influence on the sorption of diuron. American-Eurasian Journal of Agricultural and Environmental Sciences, 8, 773–778.
Ahangar, A. G., Smernik, R. J., Kookana, R. S., & Chittleborough, D. J. (2008). Clear effects of soil organic matter chemistry, as determined by NMR spectroscopy, on the sorption of diuron. Chemosphere, 70, 1153–1160.
Ahmad, R., Kookana, R. S., Alston, A. M., & Skjemstad, J. O. (2001). The nature of soil organic matter affects sorption of pesticides. I. Relationships with carbon chemistry as determined by 13C-CPMAS NMR spectroscopy. Environmental Science and Technology, 35, 878–884.
Ahmad, R., Nelson, P. N., & Kookana, R. S. (2006). The molecular composition of soil organic matter as determined by 13C NMR and elemental analyses and correlation with pesticide sorption. European Journal of Soil Science, 57, 883–893.
Berns, A., Vinken, R., Bertmer, M., Breitschwerdt, A., & Schaeffer, A. (2005). Use of 15N-depleted artificial compost in bound residue studies. Chemosphere, 59, 649–658.
Berns, A. E., Conte, P., Philipp, H., Witte, E. G., & Lewandowski, H. (2009). Interactions between 2-aminobenzothiazole and natural organic matter as evidenced by CPMAS 15N-NMR spectroscopy. Vadose Zone Journal, 8, 670–676.
Boivin, A., Cherrier, R., & Schiavon, M. A. (2005). Comparison of five pesticides adsorption and desorption processes in thirteen contrasting field soils. Chemosphere, 61, 668–676.
Briggs, G. G. (1981). Theoretical and experimental relationship between soil adsorption, octanol-water partition coefficient, water solubilities and bioconcentration factors and the parachor. Journal of Agricultural and Food Chemistry, 29, 1050–1059.
Cellis, R., Cornejo, J., Hermosin, A., & Koskimen, W. C. (1997). Sorption desorption of atrazine and simazine by model soil colloidal components. Soil Science Society of America Journal, 61, 436–443.
Chefetz, B., Bilkis, Y. I., & Polubesova, T. (2004). Sorption-desorption behaviour of atrazine and phenylurea herbicides in Kishon river sediments. Water Research, 38, 4383–4394.
Chefetz, B., Deshmukh, A. P., Hatcher, P. G., & Guthire, E. A. (2000). Pyrene sorption by natural organic matter. Environmental Science and Technology, 34, 2925–2930.
Chilom, G., & Rice, J. A. (2013). Organic pollutants in the environment. eMag Research, 2, 587–596. doi:10.1002/9780470034590.emrstm1346.
Chin, Y. P., Aiken, G. R., & Danielsen, K. M. (1997). Binding of pyrene to aquatic and commercial humic substances: the role of molecular weight and aromaticity. Environmental Science and Technology, 31, 1630–1635.
Chiou, C. T. (1989). Theoretical considerations of the particle uptake of nonionic compounds by soil organic matter. In B. L. Sawhney, & K. Brown (Eds.), Reaction and movement of organic chemicals in soils (pp. 1–29). Madison, WI: Soil Science Society of America.
Chiou, C. T., McGroddy, S. E., & Kile, D. E. (1998). Partition characteristics of polycyclic hydrocarbons on soils and sediments. Environmental Science and Technology, 32, 264–269.
Giles, C. H., McEvans, T. H., Nakhwa, S. N., & Smith, D. (1960). Studies in adsorption. Part XI. A system of classification of adsorption isotherms and its use in diagnosis of desorption mechanism and measurement of specific surface areas of solids. Journal of the Chemical Society, 3, 3973–3993.
Gunasekara, A. S., & Xing, B. (2003). Sorption and desorption of naphthalene by soil organic matter: importance of aromatic and aliphatic components. Journal of Environmental Quality, 32, 240–246.
Kang, S. H., & Xing, B. S. (2005). Phenanthrene sorption to sequentially extracted soil humic acids and humins. Environmental Science and Technology, 39, 134–140.
Kasozi, G. N., Nkedi-Kizza, P., Li, Y., & Zimmerman, A. R. (2012). Sorption of atrazine and ametryn by carbonatic and non-carbonatic soils of varied origin. Environmental Pollution, 169, 12–19.
Kulikova, N. A., & Perminova, I. V. (2002). Binding of atrazine to humic substances from soil, peat, and coal related to their structure. Environmental Science and Technology, 36, 3720–3724.
LeBoeuf, E. J., & Weber, W. J. (1997). A distributed reactivity model for sorption by soils and sediments. 8. Sorbent organic domains: discovery of a humic acid glass transition and an argument for a polymer-based model. Environmental Science and Technology, 31, 1697–1702.
Lima, D. L. D., Schneider, R. J., Scherer, H. W., Duarte, A. C., Santos, E. B. H., & Esteves, V. I. (2010). Sorption-desorption behavior of atrazine on soils subjected to different organic long-term amendments. Journal of Agricultural and Food Chemistry, 58, 3101–3106.
Mao, J. D., Hundal, S., Thompson, M. L., & Schmidt3Rohr, K. (2002). Correlation of poly(methylene)3rich amorphous aliphatic domain in humic substances with sorption of a nonpolar organic contaminant, phenanthrene. Environmental Science and Technology, 36, 929–936.
Pan, B., Xing, B., Tao, S., Liu, W., Lin, X., Xiao, Y., Dai, H., Zhang, X., Zhang, Y., & Yuan, H. (2007). Effect of physical forms of soil organic matter on phenanthrene sorption. Chemosphere, 68, 1262–1269.
Salloum, M. J., Chefetz, B., & Hatcher, P. G. (2002). Phenanthrene sorption by aliphatic-rich natural organic matter. Environmental Science and Technology, 36, 1953–1958.
Sawhney, B. L., & Singh, S. S. (1997). Sorption of atrazine by Al- and Ca-saturated semectite. Clay and Clay Minerals, 45, 333–338.
Simpson, M. J., & Johnson, P. C. E. (2006). Identification of mobile aliphatic domains in soil humin by solid state 13C-nuclear magnetic resonance. Environmental Toxicology and Chemistry, 15, 52–57.
Singh, N., Berns, A. E., Hennecke, D., Hoerner, J., Koerdel, W., & Schaeffer, A. (2010). Effect of soil organic matter chemistry on sorption of trinitrotoluene and 2,4-dinitrotoluene. Journal of Hazardous Materials, 173, 343–348.
Singh, N., & Singh, S. B. (2012). Sorption-desorption behavior of metsulfuron-methyl and sulfosulfuron in soils. Journal of Environmental Science and Health, B47, 168–174.
Sondhia, S. (2009). Leaching behaviour of metsulfuron in two texturally different soils. Environmental Monitoring and Assessment, 154, 111–115.
Tahir, N. M., & Sing, N. Y. J. (2007). Adsorption of metsulfuron–methyl on soils under oil palm plantation: a case study. Journal of Teknologi, 47, 35–43.
Tahir, N. M., Hui, T. J., Ariffin, M. M., Suratman, S., & Khoon, T. S. (2008). Adsorption of chlorimuron–ethyl and metsulfuron–methyl on selected Selangor agricultural soils. The Malaysian Journal of Analytical Science, 2, 341–347.
Wang, Q., Yang, W., & Liu, W. (1999). Adsorption of acetanilide herbicides on soils and its correlation with soil properties. Pesticide Science, 55, 1103–1108.
Wang, X., & Xing, B. (2007). Sorption of organic contaminants by biopolymer derived chars. Environmental Science and Technology, 41, 8342–8348.
Wauchope, R. D., Yeh, S., Linders, J. B. H. J., Kloskowski, R., Tanake, K., Rubin, B., Katayam, A., Koerdel, W., Gerstl, Z., Lane, M., & Wnsworth, J. B. (2002). Pesticide soil sorption parameters: theory, measurement, uses, limitations and reliability. Pest Management Science, 58, 419–445.
Xing, B. (2001). Sorption of naphthalene and phenanthrene by soil humic acids. Environmental Pollution, 111, 303–309.
Xing, B., & Pignatello, J. J. (1997). Dual-mode sorption of low-polarity compounds in glassy poly(vinyl chloride) and soil organic matter. Environmental Science and Technology, 31, 792–799.
Zhang, W., & Wang, J. J. (2007). Effect of solution pH and simulated acid rain on the behaviour of two sulfonyl urea herbicides in soil. Ying Yong Sheng Tai Xue Bao, 18, 613–619.
Zhang, G., Liu, X., Sun, K., He, Q., Qian, T., & Yan, Y. (2013). Interaction of simazine, metsulfuron-methyl and tetracycline with biochars and soils: a function of molecular structure. Journal of Soils and Sediments, 13, 1600–1611.
Compliance with Ethics Guidelines
Conflict of Interest
The authors declare that they have no competing interests.
Human and animal rights and informed consent
Consent to publish the work from co-author and the responsible authority of institution, where work was carried out, has been obtained. The research does not involve humans or animals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dutta, A., Mandal, A., Manna, S. et al. Effect of organic carbon chemistry on sorption of atrazine and metsulfuron-methyl as determined by 13C-NMR and IR spectroscopy. Environ Monit Assess 187, 620 (2015). https://doi.org/10.1007/s10661-015-4837-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4837-3