Abstract
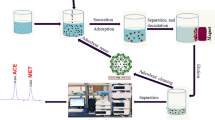
In the present study, silica magnetite mesoporous nanoparticles functionalized with a new chelating agent were synthesized and introduced as a magnetic solid phase for preconcentration of trace amounts of Cu2+, Ni2+, and Co2+ ions from aqueous solutions. Briefly, MCM-41 mesoporous-coated magnetite nano-particles (MMNPs) with particle size lower than 15 nm were synthesized via chemical co-precipitation methods. Then, N-(4-methoxysalicylidene)-4,5-dinitro-1,2-phenylenediamine (HL) as a new chelating agent was synthesized and used for surface modification of mesoporous magnetic solid phase by dispersive liquid-liquid functionalization (DLLF) as a new rapid method to form HL functionalized mesoporous magnetite nanoparticles (MMNPs─HL). The structure and morphology of prepared sorbent were characterized by FT-IR, XRD, VSM, and TEM. Finally, the prepared nanoparticles were utilized for preconcentration of Cu2+, Ni2+, and Co2+ ions prior to determination by atomic absorption spectrophotometery. The calibration graph was obtained under the optimized conditions with linear dynamic range of 1.0–300 μg L−1 and correlation coefficient (r 2) of 0.998. The detection limits of this method for cobalt, nickel, and copper ions were 0.03, 0.03, and 0.04 ng/mL, respectively. Finally, the method was successfully applied to the extraction and determination of the analyte ions in natural waters and reference plant samples.

ᅟ










Similar content being viewed by others
References
Azevedo Lemos, V., Selis Santos, M., Silva dos Santos, M. J., Rodrigues Vieira, D., & Novaes, C. G. (2007). Determination of copper in water samples by atomic absorption spectrometry after cloud point extraction. Microchimica Acta, 157, 215–222.
Cerrato Oliveros, M. C., de Blas, O. J., Pav’on, J. L. P., & Cordero, B. M. (1998). Cloud point preconcentration and flame atomic absorption spectrometry: application to the determination of nickel and zinc. Journal of Analytical Atomic Spectrometry, 13, 547–550.
Chrastny, V., & Komarek, M. (2009). Copper determination using ICP-MS with hexapole collision cell, J. Chemical Papers. Journal Chemical Papers, 24, 512–521.
Donati, G. L., Nascentes, C. C., Nogueira, A. R. A., Arruda, M. A. Z., & N’obrega, J. A. (2006). Acid extraction and cloud point preconcentration as sample preparation strategies for cobalt determination in biological materials by thermospray flame furnace atomic absorption spectrometry. Microchemical, 82, 189–195.
Golshekan, M., & Shariati, S. (2013). Dispersive liquid–liquid microextraction of copper ions as neocuproine complex in environmental aqueous samples. Acta Chimica Slovenica, 60, 358–367.
Golshekan, M., Shariati, S., & Saadatjoo, N. (2014). The synthesis of aminonaphtols and β-amino carbonyls in the presence of a magnetic recyclable Fe3O4@MCM-48-NaHSo4 nano catalyst. RSC Advances, 4, 16589–16596.
Greenwood, N. N., & Earnshow, A. (1984). Chemistry of elements. New York: Pergamon.
Hamley, I. W. (2003). Nanostructure fabrication using block copolymers. Nanotechnology Nanotechnology, 14, 4484–4500.
Jang, J. H., & Lim, H. B. (2010). Characterization and analytical application of surface modified magnetic nanoparticles. Microchemical, 94, 148–158.
Khuhawar, M. Y., Sarafraz Yazdi, A., & Uden, P. C. (2006). Platinum-selective capillary gas chromatographic determination with microwave-induced plasma atomic emission detection. Chromatographia, 21, 223–229.
Kim, Y., Lee, B., & Yi, J. (2003). Preparation of Functionalized Mesostructured Silica Containing Magnetite (MSM) for the removal of copper ions in aqueous solutions and its magnetic separation. Separation Science and Technology, 38, 2533–2548.
Manzoori, J. L., & Karim-Nezhad, G. (2004). Development of a cloud point extraction and preconcentration method for Cd and Ni prior to flame atomic absorption spectrometric determination. Analitica Chimica Acta, 521, 173–177.
Mohammadi, S. Z., Hamidian, H., Karimzadeh, L., & Moeinadini, Z. (2013). Simultaneous extraction of trace amounts of cobalt, nickel and copper ions using magnetic iron oxide nanoparticles without chelating agent. Journal of Analytical Chemistry, 68, 953–958.
Pankhurst, Q. A., Connolly, J., Jones, S. K., & Dobson, J. (2003). Applications of magnetic nanoparticles in biomedicine. Journal of Physics D: Applied Physics, 36, 3727–3731.
Rahchamani, J., Behzad, M., Bezaatpour, A., Jahed, V., Dutkiewicz, G., Kubicki, M., & Salehi, M. (2011). Oxidovanadium complexes with tetradentate Schiff bases: Synthesis, structural, electrochemical and catalytic studies. Polyhedron, 30, 2611–2618.
Saadatjoo, N., Golshekan, M., Shariati, S., Kefayati, H., & Azizi, P. (2013). Organic/inorganic MCM-41 magnetite nanocomposite as a solid acid catalyst for synthesis of benzo[α]xanthenone derivatives. Journal of Molecular Catalysis A: Chemical, 377, 173–179.
Sasmaz, A., & Yaman, M. (2006). Distribution of chromium, nickel, and cobalt in different parts of plant species and soil in mining area of Keban. Turkey Communications in Soil Science and Plant, 37, 1845–1857.
Sawyer, C. N., McCarty, P. L., & Parkin, G. F. (1996). Chemistry for environmental engineering, 4th ed, McGraw-Hill, p 634.
Scheinberg, I. H., Morell, P., & Ceruloplasmin, A.G. in: G.L. Eichhorn (Ed.). (1973). Inorganic biochemistry. Elsevier. New York. 1, 306-343.
Shin, S., & Jang, J. (2007). Thiol containing polymer encapsulated magnetic nanoparticles as reusable and efficiently separable adsorbent for heavy metal ions. Chemical Communications, 27, 4230–4232.
Soler-Illia, G. J. A. A., Sanchez, C., Lebeau, B., & Patarin, J. (2002). Chemical strategies to design textured materials: from microporous and mesoporous oxides to nanonetworks and Hierarchical structures. Chemical Reviews, 102, 4093–4138.
Sousa, E. M. B., Doadrio, A. L., Doadrio, J. C., Perez-Pariente, J., Izquierdo-Barba, I., & Vallet-Regi, M. (2004). Mesoporous SBA-15 HPLC evaluation for controlled gentamicin drug delivery. Journal of Controlled Release, 97, 3659–3663.
Soylak, M., Elci, L., & Dogan, M. (1997). Determination of trace amounts of cobalt in natural water samples as 4-(2-thiazolylazo) recorcinol complex after adsorptive preconcentration. Analytical Letters, 30, 623–631.
Soylak, M., Elci, L., Narin, I., & Dogan, M. (2001). Application of solid-phase extraction for the preconcentration and separation of trace amounts of cobalt from urine. Trace Element Eletroly, 18, 26–29.
Xie, L., Jiang, R., Zhu, F., & Liu, H. (2014). Application of functionalized magnetic nanoparticles in sample preparation. Analytical and Bioanalytical Chemistry, 406, 377–399.
Yamini, Y., & Tamaddon, A. (1999). Solid-phase extraction and spectrophotometric determination of trace amounts of copper in water samples. Talanta, 49, 119–124.
Acknowledgments
The authors acknowledge the Young Researchers and Elite Club, Lahijan Branch, Islamic Azad University, Lahijan, Iran for supporting this work.
Compliance with ethical standards
We confirm that this study did not involve human participants and/or animals. We confirm that there are no known conflicts of interest associated with this publication. In addition, we guarantee that the work reported in the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. Also, we further confirm that the order of authors listed in the manuscript has been approved by all of us.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azizi, P., Golshekan, M., Shariati, S. et al. Solid phase extraction of Cu2+, Ni2+, and Co2+ ions by a new magnetic nano-composite: excellent reactivity combined with facile extraction and determination. Environ Monit Assess 187, 185 (2015). https://doi.org/10.1007/s10661-015-4419-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4419-4