Abstract
The principal objective of the current study was to elucidate the potential influence of acid mine drainage (AMD) pond on neighboring farmer's wells in the Podwiśniówka area (south-central Poland), using North American Shale Composite (NASC)-normalized rare earth element (REE) concentration profiles. The well waters generally displayed a distinctly positive Eu anomaly similar to that of parent rocks and AMD sediment. In contrast, the AMD pit pond water exhibited the typical roof-shaped NASC-normalized REE concentration pattern with a strong positive Gd anomaly. The low pH (mean of 2.9) of this pond water is induced by oxidation of pyrite that occurs in quartz veins and rocks exposed in the abandoned Podwiśniówka quarry. The principal source of REEs in turn is a crandallite series of aluminum–phosphate–sulfate (APS) minerals (gorceixite with florencite and Ce-bearing goyazite) that prevail in most clayey shales. These data indicate that the REE contents of the AMD pit pond and well waters are linked to bedrock mineralogy and lithology, but not to pyrite mineralization. The diverse REE patterns of NASC-normalized REE concentrations of the AMD and well waters may suggest complex sorption and desorption processes that occur at the rock–water interface influenced by different pH, Eh, temperature, and other factors. This is evidenced by a presence of strong positive Ce anomaly in the rocks, a lack of Ce anomaly in the AMD water and sediment, and the dominant negative anomaly of this element in the well waters. Variations in correlation coefficients (r 2) of REE concentrations between the rocks and the well waters may also result from a different contribution of quartzites, clayey shales, or tuffites to the REE signal of well waters as well as from mixing of shallow groundwater with infiltrating rainwater or meltwater with different REE profiles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lanthanoids or lanthanides, including La (Z = 57) to Lu (Z = 71), are usually assigned to rare earth elements (REEs), especially in geologic literature. According to the International Union of Pure and Applied Chemistry, Y (Z = 39) and even Sc (Z = 21) are also referred to as REEs (Connelly et al. 2005). Except for Ce (Ce3+, Ce4+) and Eu (Eu2+, Eu3+), they occur in a trivalent state in surface environmental systems and their ionic radii decrease with an increase of atomic number from 103 pm in La3+ to 86 pm in Lu3+ (“lanthanide contraction”). This chemical property is a basis for dividing REEs into two main groups: (1) light REEs (LREEs) including La through Eu or Gd and (2) heavy REEs (HREEs) from Gd or Tb through Lu. Some authors also discriminate the third group that partly covers LREEs and HREEs: medium REEs (MREEs) that encompass Sm through Ho.
The REEs have found an application in studying meteoritic and terrestrial materials for a few decades (e.g., Rasmussen et al. 1998; Friedrich et al. 2002; Bolhar et al. 2005; Quinn et al. 2006; Bouvier et al. 2008; Hu and Gao 2008). These elements have also been used in investigating a variety of environmental and biological samples, particularly in pinpointing geologic and anthropogenic sources. These studies also included water–rock interactions as well as fractionation and speciation of dissolved and suspended REEs in aquatic systems in both aerobic and anaerobic conditions (e.g., Markert et al. 1989; Nozaki et al. 2000; Gaillardet et al. 2003; Dubinin 2004; Gammons et al. 2005a b; Han and Liu 2006; Sultan and Shazili 2009; Dubinin and Rimskaya-Korsakova 2011). One of these examples is Gd, which has recently been recognized as an anthropogenic micropollutant (e.g., Knappe et al. 2005; Bau et al. 2006; Kulaksiz and Bau 2011). Of particular interest are acid mine drainage (AMD) streams, ponds, and lakes that give a direct insight into local and regional geologic sources highlighted by a number of REE profiles with LREE or HREE enrichments or MREE depletion (e.g., Johannesson and Lyons 1995; Gimeno et al. 2000; Worrall and Pearson 2001; Bozau et al. 2004; Verplanck et al. 2004; Gammons et al. 2005b). These studies have also showed that in low-temperature surface environments, REEs behave differently in relation to aqueous complexation, ion sorption–desorption, and mineral precipitation–dissolution or transformation. All these processes lead to REE fractionation in various environmental compartments, especially in surface waters (e.g., Tricca et al. 1999; Steinmann and Stille 2008).
An increased number of REE case studies has been possible due to rapid developments in mass spectrometry methods using single-collector sector-field or quadrupole inductively coupled plasma mass spectrometer (ICP-MS) and multi-collector inductively coupled plasma mass spectrometer (MC-ICP-MS) along with isotope dilution mass spectrometry (IDMS) (e.g., Lichte et al. 1987; Baker et al. 2002; Kent et al. 2004; Merten and Büchel 2004; Wieser and Schwieters 2005).
In order to compare REE concentrations in different geologic and environmental materials, the results derived from these element measurements are commonly normalized to chondrite (Boynton 1984), North American Shale Composite (NASC) (Haskin et al. 1968; Gromet et al. 1984), or Post-Archean Australian Shale (PAAS) (McLennan 1989; Pourmand et al. 2012). Chondrite is used in studying endogenous processes whereas “shales” are applied to study hypergene processes and are commonly utilized in environmental studies.
The principal objective of this case study was to assess the anthropogenic (Podwiśniówka acid pit pond) or geogenic (bedrock mineralogy and lithology) influence on the waters of farmer's wells located in the neighboring area using REEs as geochemical tracers. For this purpose, the well and AMD waters and AMD sediment were analyzed for REEs and additionally Y contents. This enabled us to evaluate a contribution of bedrock mineralogy and lithology as well as sorption and desorption to the chemistry of well waters.
Study area
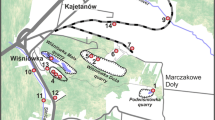
The study area covered Podwiśniówka Mt., which is located in the easternmost part of the dismembered Wiśniówka massif of the western part of the Holy Cross Mountains (Fig. 1a). The entire area is situated within the uppermost Middle Cambrian (?) and Upper Cambrian (Furongian) series composed of quartzites, quartzitic sandstones, and siltstones, in places with clayey shale, tuffite, and bentonite interbeds. The Wiśniówka massif is characterized by a complex tectonic structure with intense folding and faulting. NE–SW to N–S trending faults prevail over parallel faults (e.g., Żylińska et al. 2006 and references therein). The foot of Podwiśniówka Mt. is blanketed by Pleistocene deposits that include sandy–clayey tills (predominantly in the northern and eastern parts) and loesses and sandy loesses (mostly in the southern part).
Petrographic studies of the host rocks in the Podwiśniówka quarry have indicated that quartz and muscovite are predominant rock-forming minerals of the quartzites, clayey shales, and tuffs. Most of the clayey shales contain a considerable amount of aleuritic terrigenous and volcanogenic quartz grains. Smectite, kaolinite, and pyrite (including As-rich variety) are subordinate, whereas chalcedony and feldspars occur only in trace amounts. The Podwiśniówka acid pit pond sediment consists of quartz, illite, chlorite, and kaolinite with some admixture of jarosite and probably subordinate fougérite and mixed-layered illite–smectite (Migaszewski et al. 2007b, 2008, 2013).
There is an abandoned quarry at the top of this towering mountain. Extraction of quartzites for manufacturing crushed aggregates in the early 1990s of the twentieth century exposed a quartz–pyrite zone, which, along with a lack of buffering rocks and gangue minerals, have led to generation of unique acid mine drainage. The AMD process is also enhanced by the presence of crypto- to microcrystalline As-bearing pyrite being the only sulfide mineral that occurs in this area. The pit floor is filled with a shallow acid pond about 1.5 m deep (Fig. 1b), which is highlighted by a low pH varying from 1.7 to 3.6 and exceptionally low concentrations of soluble sulfates, iron, and other elements compared to other different AMD areas throughout the world. These combined physicochemical and chemical parameters make this water body unique among AMD ponds (Migaszewski et al. 2007a, 2008).
The foot of Podwiśniówka Mt. is surrounded by two villages Masłów II and Marczakowe Doły (Fig. 1a). Some houses possess wells that provide inhabitants with potable water.
Materials and methods
Fieldwork and sampling
Fieldwork was performed on September 13, 2011 and on March 13 and June 29, 2012 during the sunny weather with a temperature range of about 15–20 °C. This included direct measurements of pH, electric conductivity (EC), and temperature (T) of water, using a pH-meter CP-103 and an EC-meter CC-101 (Elmetron, Poland). In addition, alkalinity and concentrations of Fetotal, Fe2+, SO4 2−, Cl−, NO3 −, NO2 −, and NH4 + were determined on-site using a field spectrophotometer LF-205 (Slandi, Poland). Fe(III) was calculated as the difference between Fetotal and Fe(II). Detection limits expressed as milligrams per liter are given in parentheses: Fe2+ (0.1), Fetotal (0.2), SO4 2− (10), Cl− (2.0), NO3 − (0.5), NO2 − (0.02), and NH4 + (0.1).
Ten farmer's wells and the ADM pit were visited during all three sampling campaigns. In all, 11 water samples were collected during each series of measurements. The water samples for REE measurements were placed in polyethylene bottles and cooled at a temperature of about 4 °C. In addition, one surface sediment sample from the Podwiśniówka acid pit pond was collected for REE determinations. This sample consisted of several subsamples sieved on-site in water (Tarvainen and Salminen 1997). The fraction which passed a 0.15-mm sieve was retained for analysis.
During sample collection, transport, storage, and preparation, procedures were followed to minimize the possibility of contamination. A set of water samples included one blank and two replicates for each sampling campaign.
Sample preparation and chemical analysis
The water samples were filtered through 0.45-μm pore-sized filters whereas the sediment sample was dried at an ambient temperature of about 16 °C, then disaggregated to pass a <0.063-mm sieve using a Fritsch's blender, and subsequently digested in aqua regia using a closed microwave system. All the samples were analyzed for 14 REEs (La through Lu) and Y using an ICP-MS instrument (model ELAN DRC II, Perkin Elmer). Operating conditions were as follows: sweeps/reading–20, readings/replicate–3, replicates–4, nebulizer gas flow–0.99 L min−1, plasma gas flow–1.5 L min−1, lens voltage–6.50 V, plasma power–1,275 W. Correction equations for Nd, Sm, Gd, Dy, and Yb were used for elimination of interferences. The ICP-MS instrument was optimized with a standard daily procedure. For REE determination, a series of Multi-element Calibration Standard 2 Perkin Elmer solutions was used (0.1, 0.5, 1, 10, 25, 50, and 100 μg L−1).
The geologic multi-element reference materials applied for measuring element concentrations in the samples were (1) PPREE1 and SCREE1 (Table 2 in Verplanck et al. (2001)) for waters and (2) Certified Reference Material (CRM) Tibet Sediment NCS DC 70317 for AMD sediment. For comparison, the REE concentrations derived from ICP-MS measurements were normalized to NASC using values given by Haskin et al. (1968) and Gromet et al. (1984). Quality control included both accuracy (CRM) and precision (triplicates). The average recovery of elements from the CRM was 96 %, and the uncertainty of the method was below 10 %. The chemical analyses of waters were performed in the Geochemical Laboratory of the Institute of Chemistry, Jan Kochanowski University in Kielce.
The pyrite and rock samples (clayey shales, quartzites, tuffs) were dried at ambient temperature (about 16 °C) and then disaggregated to pass a <0.063-mm sieve using an agate Fritsch's blender Pulverisette 5. After that, the samples were digested with HF–HNO3–HClO4. Determinations were made for La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, and Yb (Lu was used as an internal reference material for determining REEs) using ICP-MS (ELAN DRC II spectrometer, Perkin Elmer). The chemical analyses were performed according to methods and techniques used for geologic materials in the Central Chemical Laboratory of the Polish Geological Institute–State Research Institute in Warsaw.
Results and discussion
Geochemistry of waters
The average values of selected physicochemical and chemical parameters of farmer's well and AMD waters in the Podwiśniówka area are presented in Table 1. The farmer's well waters examined are characterized by a pH in the range of 5.3 to 7.3, low EC (234 to 540 μS cm−1) and alkalinity (24 to 162 mg CaCO3 mg L−1), and relatively low concentrations of SO4 2− (<10 to 172 mg L−1), Cl− (2 to 10 mg L−1), NO3 − (1 to 23 mg L−1), NO2 − (<0.02 to 0.10 mg L−1), and NH4 + (<0.1 to 1.0 mg L−1). Of these physicochemical and chemical parameters, the contents of NH4 + were in most wells close to or even above allowable limits for drinking waters, i.e., 0.5 mg L−1 (Ministry of Health 2007). Apart from well W7, the concentrations of Fe3+ (0.8 mg L−1) were below detection limits for a given method, i.e., <0.2 mg L−1. Well W8 also exhibited the lowest pH (5.3) and simultaneously the highest contents of SO4 2− (172 mg L−1). No significant seasonal variations of water chemistry in individual wells were noted.
In contrast, the acid pit pond water revealed a distinctly lower pH averaging 2.9; a higher EC (mean of 765 μS cm−1); higher concentrations of Fe2+ (3.0 mg L−1), Fe3+ (3.8 mg L−1), and SO4 2− (150 mg L−1); and an alkalinity below 0.1 CaCO3 mg L−1. The other chemical parameters (Cl−, NO3 −, NO2 −, NH4 +) were measured in June 29, 2012, and except for Cl− (60.0 mg L−1), they were similar to those of well waters. It is interesting to note that nearly the same values were reported by the previous AMD studies conducted in May 2004 (Migaszewski et al. 2007a, 2008).
The principal source of SO4 2− ions in these waters is oxidation of pyrite that occurs in the form of veins in quartzites or scattered inclusions and aggregates in most clayey shales. The previous geochemical and isotopic studies carried out in the Holy Cross Mountains indicated that atmospheric sulfur deposition was significantly reduced during the last decade (Migaszewski et al. 2007a, 2008; Michalik and Migaszewski 2012). Variations in the pH of well waters are, in turn, closely linked to a state of equilibrium between two competing ions: SO4 2− and HCO3 − derived from calcite-bearing loess and till patches. It is interesting to note that the only correlation (r 2 = 0.60) was found between the pH and the SO4 2− concentrations. The near-neutral pH combined with very low contents of Fe2+ (<0.1 mg L−1) and Fe3+ (<0.2–0.8 mg L−1) in these wells points up to oxidation and precipitation of iron at a higher pH in a groundwater circulation system. Cl− ions occur in all the waters examined and are primarily of geologic and sea aerosol origin. However, during the winter, the main load of Cl− contamination may be derived from NaCl used for deicing of local roads (Gałuszka et al. 2011). The highest concentrations of NO3 − were found in wells which were either abandoned or only occasionally used (W1, W2, W4, W6, W9). Infrequent drawing of water has brought about seasonal blooming of algae which are a source of nitrogen compounds. Some differences in water chemistry between the wells resulted from overlapping various geologic, biological, and to a lesser extent anthropogenic (sewage, manure, and fertilizers) sources, mediated by environmental factors.
REEs in waters
The measured concentrations of individual REEs, Y, total REEs, LREEs, HREEs, MREEs, as well as LREE/HREE ratios and selected not normalized and NASC-normalized element pairs are presented in Table 2. Because of the use of 0.45-μm pore-sized filters, these contents reflect both true dissolved and partly colloidal REE species. The total REE concentrations of AMD water samples varied at least 5 orders of magnitude in relation to well water samples. This is due to the presence of exposed REE-bearing rocks in the Podwiśniówka stone pit. However, it is noteworthy that the contents of REEs in the pit pond water were not too high (mean of 24.90 μg L−1) compared to those of other AMD sites throughout the world, for example, 457.73 μg L−1 in the natural standard reference water sample PPREE1 (Verplanck et al. 2001) or in the range of hundreds of micrograms per liter in southern Tuscany, central Italy (Protano and Riccobono 2002), or the upper Rio Agrio in northern Patagonia, Argentina (Gammons et al. 2005b). The measured total REE concentrations in the well waters were also low, varying from 0.375 (W5) to 5.33 μg L−1 (W6). They were similar to those (range of <0.005 to 11.540 μg L−1) from about 500 stream waters of Eastern Canada (Leybourne and Johannesson 2008). The well waters also exhibited much lower levels of individual REEs and Y than the pit pond water impacted by AMD. Of the measured REEs, Nd was a predominant element in the waters of wells W1, W3, W4, W6, and W8–10 (0.13–1.70 μg L−1), whereas Ce was abundant in the waters of wells W2, W5, and W7 (0.08–1.70 μg L−1) and the waters of the acid pit pond (7.96 μg L−1) (Table 2).
In all these water samples, the measured LREEs distinctly prevailed over HREEs with ratios in the range of 1.76 (W4) to 8.02 (W7). This is also evidenced by the La/Yb ratio, which varied from 1.46 (W4) to 11.43 (W7). The same relationship is highlighted by its NASC-normalized equivalent: 0.14–1.18 (Table 2). The NASC-normalized concentrations of LREEs, HREEs, and MREEs from three sampling campaigns are presented in Fig. 2. The NASC-normalized concentrations of HREEs always prevailed over those of LREEs and MREEs in wells W1, W3, W4, W6, and W10 and the AMD pit pond. These levels underwent seasonal changes, which is best exemplified by W4 exhibiting the highest HREE peak on September 13, 2011.
The [La/Sm]NASC ratio varied from 0.04 (W2) to 0.87 (W7) with a mean of 0.47 (Table 2). The study conducted by Leybourne and Johannesson (2008) revealed that surface waters of Eastern Canada were typically LREE-depleted with a [La/Sm]NASC ratio in the range of 0.16 to 5.84 and a mean of 0.60. This and other index ratios: [La/Yb]NASC (0.14 to 1.18), [Gd/Yb]NASC (0.43 to 1.50), and [Gd/Dy]NASC (1.00 to 1.67), partly overlap those obtained from the Eastern Canada study. Except for the [Gd/Yb]NASC (2.27), the remaining ratios of the Podwiśniówka pit pond water occur within ranges characteristic of the well waters examined.
The well and AMD waters displayed a bit stronger positive correlation between the pH and the HREEs (r 2 = 0.81) or MREEs (r 2 = 0.80) than between the pH and the LREEs (r 2 = 0.75). This may suggest that HREEs or MREEs are not so preferentially scavenged by clay minerals, iron oxyhydrosulfates and oxyhydroxides, or goethite, as indicated by previous studies (e.g., Coppin et al. 2002; Borrego et al. 2005; Romero et al. 2010).
Plots of NASC-normalized REE patterns of water samples from the ten farmer's wells and the acid pit pond collected in three series are depicted in Fig. 3. Most wells revealed distinctly positive Eu anomalies, especially W7 on September 13, 2011 and W10 on June 29, 2012. An exception was W4 that additionally exhibited stronger positive Er, Tm, Yb, and Lu anomalies, particularly on September 13, 2011 and June 29, 2012. In contrast, on March 13, this well displayed a stronger positive Eu anomaly. The waters of W1, W3, W4, W6, W8, and W10 showed a distinctly negative Ce anomaly during three sampling campaigns.
In general, the well waters showed seasonal variations in the REE profiles, for example, on March 13, 2012, the water of W1 had a stronger positive Gd anomaly associated with positive excursions of Eu, Er, Sm, Nd, and Lu. In contrast, on September 13, 2011, the REE profile was highlighted by positive Lu, Tm, and Eu anomalies, whereas on June 29, 2012, Eu and Lu superseded the other REEs. Even a more complex REE pattern was observed in the water of W6. A REE profile of September water with weak excursions of Lu, Gd, and Eu was superseded by diverse patterns with positive Gd, Sm, Nd, Er, Eu, and Yb anomalies in March and positive Eu, Gd, Tb, Sm, and Lu anomalies combined with strong negative Ce and Er anomalies in June. At this stage of study, it is difficult to pinpoint the immediate cause of these seasonal variations. They may be brought about by mixing of shallow groundwater with infiltrating rainwater or meltwater that carry away weathering waste material of different chemistry and mineralogy.
The shale-normalized REE concentration profile of the AMD water differed considerably from that of the well waters and was highlighted by strong positive anomalies of HREEs and MREEs, but especially by a Gd peak. Compared to the well waters examined, the REE roof-shaped pattern remained unchanged during three measurement series (Fig. 4a). Somewhat lower REE concentrations on March 13, 2012 resulted from the presence of ice cover that prevented supplying REE loads from exposed pit walls. However, the overlapping shapes of three plots suggest that there have been no dramatic seasonal variations in the distribution pattern of REE concentrations of the Podwiśniówka AMD water.
Sources of REEs in waters
The NASC-normalized REE patterns of rocks and pyrite that crop out in the abandoned Podwiśniówka quarry are presented in Fig. 4b. The pyrite, which is the main source of acidity and sulfate in the AMD pit pond water, is both trace element- and REE-depleted (Migaszewski et al. 2007a, 2008). Shale-normalized concentrations of REEs show a flat profile with the predominance of LREEs over HREEs. In contrast, the bulk rocks contain the highest concentrations of REEs with distinctly positive Ce, Eu and Sm anomalies. The principal source of these elements is a crandallite series, of which gorceixite BaAl3H[(OH)6(PO4)2] is a predominant mineral, whereas florencite (REE)Al3(PO4)2(OH)6 and goyazite SrHAl3[(PO4)2(OH)6] as well as REE phosphates, such as xenotime Y[PO4], are scarce (Migaszewski et al. 2007b; Migaszewski and Gałuszka 2010). This is evidenced by a strong correlation (r 2) between the sum of dissolved REE concentrations in the AMD pit pond water and the sum of total REE concentrations in the source rocks (0.92).
The characteristic roof-shaped NASC-normalized REE concentration pattern of the Podwiśniówka pit pond water with the predominance of Gd may suggest that MREEs are either more easily leached from the parent rocks or more soluble under the pH and redox conditions that prevail in AMD ponds and lakes (Leybourne et al. 2000). There may be another explanation for the convex-up REE pattern. These elements may occur in the water as two basic chemical species: (1) with the predominance of free metal cations (Leybourne et al. 2000) and/or (2) sulfate complexes LnSO4 + (Johannesson and Lyons 1995). In contrast to the REE sulfate complexes (especially from Tb through Lu), the partitioning of soluble REEs into free metal species (preferentially La3+ through Gd3+) rises with an increase of atomic number as indicated by the speciation study by Zhao et al. (2007).
Compared to the acid water, the Podwiśniówka pit pond sediment exhibits a different pattern of shale-normalized REE concentrations. This shows a strong positive Eu anomaly associated with somewhat less positive Sm, Gd, and Nd anomalies. Moreover, except for the lack of positive Ce anomaly, this pattern is generally similar to that of bedrock (Fig. 4b). The total concentrations of REEs and Y in the sediment were 103.7 mg kg−1 and 5,216.4 μg kg−1, respectively. The former contents were within the range of those (49.8–202 mg kg−1) recorded in AMD sediments from the Sitai coal mine, China (Zhao et al. 2007). The comparison of NASC-normalized REE distribution patterns of bulk rocks, sediment, and water of the Podwiśniówka quarry provides an opportunity of a direct insight into geochemical interactions between the sediment and the water column. As opposed to Ce, Gd appears to be more easily leached from rocks by rainwater or meltwater containing dissolved acidic products of pyrite oxidation. However, different NASC-normalized REE profiles of both AMD water and sediment, but particularly the presence of positive Gd vs. Eu anomaly, may be indicative of greater mobility of Gd as well as immobilization of Eu by the sediment. It is noteworthy that the previous study of the mineralogical composition of this sediment indicated the presence of quartz, illite (−2 M1), kaolinite (−1Md), goethite, hematite, jarosite, and subordinate schwertmannite (Migaszewski et al. 2008, 2013). Some of these minerals may be potential scavengers of Eu.
Compared to the AMD environment, a circumneutral or slightly alkaline environment of well waters favors both leaching of Eu from the parent rocks and greater mobility of this element. In contrast, the distinctly negative Ce anomaly that occurs in most well waters suggests that this element may be either immobilized in the source rocks (Hall et al. 1995) or scavenged by iron oxyhydrosulfates and oxyhydroxides or clay minerals due to oxidation of Ce3+ to Ce4+. The latter chemical species shows enhanced surface reactivity in oxygenated water environments at a circumneutral pH (Fleet 1984).
The correlation coefficients (r 2) of REE concentrations between the waters of individual wells and the bedrock are presented in Table 3. The highest statistically significant correlations (≥0.85; P < 0.05) are noted between the waters of the AMD pit pond and wells W2 (0.95) and W7 (0.92). However, it should be stressed that the same relationship is found between the source rocks and farmer's wells. Due to the genetic affinity of REEs in the waters and rocks examined as well as some uncertainty of geochemical interactions, it is difficult to determine the contribution of the AMD pit pond to the chemistry of well waters. However, there are two geologic clues that back up the rock source option. They include (1) isolation of pond floor lined with sediments and (2) discharges from these and other wells exceeding the losses of pond water volume (which is primarily controlled by evaporation). Moreover, the lack of Gd anomaly in most well waters may suggest that they are mostly recharged by shallow perched aquifers.
Conclusions
The following conclusions can be drawn from the REE data derived from this study:
-
1.
The pH and sulfate concentrations of the Podwiśniówka acid pit pond water are generated primarily by oxidation of pyrite. In contrast, the exposed rock formations are a principal source of REEs.
-
2.
The AMD pit pond water shows a constant convex-up NASC-normalized REE concentration pattern with the predominance of Gd. The strong positive Gd anomaly of the acid water may indicate that the AMD environment favors substantial mobility of this element compared to other REEs.
-
3.
The AMD pond sediment exhibits the same roof-shaped NASC-normalized REE concentration pattern as the AMD water, but with a shift of peak value from Gd to Eu.
-
4.
The lack of Ce anomaly in the AMD water and sediment and the dominant negative Ce anomaly in the well waters suggest that this element is immobilized in source rocks during weathering.
-
5.
The waters of wells W1, W3, W4, W6, and W10 and the AMD pit pond are generally characterized by a significant enrichment of HREEs with respect to both LREEs and MREEs.
-
6.
The geochemical and geologic data suggest that the principal REE source of the farmer's well waters is bedrock mineralogy and lithology. The predominant positive Eu anomaly in these two media may reflect both relative mobility of this element in a circumneutral pH and a host-rock signal. In contrast, the prevalent strong negative Ce anomaly in the well waters suggests that at a circumneutral pH, this element is not leached from the parent rocks and presumably also immobilized by iron oxyhydroxides or clay minerals.
-
7.
Seasonal variations in the REE profiles of farmer's well waters may be induced by mixing of shallow groundwater with infiltrating rainwater and meltwater of different chemistry.
Considering this, the advantages of using REEs include the characteristics of waters that have a close association with bedrock mineralogy and lithology. This feature offers the possibility of reflecting geochemical anomalies in waters as well as the ability to pinpoint or exclude simultaneously geochemical interactions. In general, these results are in agreement with other data derived from the REE investigations performed at different sites throughout the world. However, to better understand different aspects of spatial and temporal distribution of REEs and their behavior under diverse physicochemical and biotic conditions, more detailed studies are needed to elucidate the contribution of individual factors.
References
Baker, J., Waight, T., & Ulfbeck, D. (2002). Rapid and highly reproducible analysis of rare earth elements by multiple collector inductively coupled plasma mass spectrometry. Geochimica et Cosmochimica Acta, 66(20), 3635–3646.
Bau, M., Knappe, A., & Dulski, P. (2006). Anthropogenic gadolinium as a micropollutant in river waters in Pennsylvania and in Lake Erie, northeastern United States. Chemie der Erde, 66, 143–152.
Bolhar, R., Kamber, B. S., Moorbath, S., Whitehouse, M. J., & Collerson, K. D. (2005). Chemical characterization of earth's most ancient clastic metasediments from the Isua Greenstone Belt, southern West Greenland. Geochimica et Cosmochimica Acta, 69(6), 1555–1573.
Borrego, J., López-González, N., Carro, B., & Lozano-Soria, O. (2005). Geochemistry of rare-earth elements in Holocene sediments of an acidic estuary: environmental markers (Tinto River Estuary, South-Western Spain). Journal of Geochemical Exploration, 86, 119–129.
Bouvier, A., Vervoort, J. D., & Patchett, P. J. (2008). The Lu-Hf and Sm-Nd isotopic composition of CHUR: constraints from unequilibrated chondrites and implications for the bulk composition of terrestrial planets. Earth Planetary Science Letters, 273(1–2), 48–57.
Boynton, W. V. (1984). Cosmochemistry of the rare earth elements: meteorite studies. In P. Henderson (Ed.), Rare earth element geochemistry. Developments in geochemistry (pp. 63–114). Amsterdam: Elsevier.
Bozau, E., Leblanc, M., Seidel, J. L., & Stärk, H.-J. (2004). Light rare earth elements enrichment in an acidic mine lake (Lusatia, Germany). Applied Geochemistry, 19, 261–271.
Connelly, N. G., Damhus, T., Hartshorn, R. M., & Hutton, A. T. (Eds.). (2005). Nomenclature of inorganic chemistry: IUPAC recommendations 2005 (p. 377). Cambridge: RSC.
Coppin, F., Berger, G., Bauer, A., Castet, S., & Loubet, M. (2002). Sorption of lanthanides on smectite and kaolinite. Chemical Geology, 182, 57–68.
Dubinin, A. V. (2004). Geochemistry of rare earth elements in the ocean. Lithology and Mineral Resources, 39(4), 289–307.
Dubinin, A. V., & Rimskaya-Korsakova, M. N. (2011). Geochemistry of rare earth elements in bottom sediments of the Brazil Basin, Atlantic Ocean. Lithology and Mineral Resources, 46(1), 1–16.
Fleet, A. J. (1984). Aqueous and sedimentary geochemistry of the rare earth elements. In P. Henderson (Ed.), Rare earth element geochemistry. Developments in geochemistry (pp. 343–373). Amsterdam: Elsevier.
Friedrich, J. M., Wang, M. S., & Lipschutz, M. E. (2002). Comparison of the trace element composition of Tagish Lake with other primitive carbonaceous chondrites. Meteoritics and Planetary Science, 37(5), 677–686.
Gaillardet, J., Viers, J., & Dupre, B. (2003). Trace elements in river waters. In D. H. Heinrich & K. T. Karl (Eds.), Treatise on geochemistry: surface and groundwater, weathering and soils (pp. 225–272). Oxford: Pergamon.
Gałuszka, A., Migaszewski, Z. M., Podlaski, R., Dołęgowska, S., & Michalik, A. (2011). The influence of chloride deicers on mineral nutrition and the health status of roadside trees in the city of Kielce, Poland. Environmental Monitoring Assessment, 176, 451–464.
Gammons, C. H., Wood, S. A., & Nimick, D. A. (2005a). Diel behavior of rare earth elements in a mountain stream with acidic to neutral pH. Geochimica et Cosmochimica Acta, 69(15), 3747–3758.
Gammons, C. H., Wood, S. A., Pedrozo, F., Varekamp, J. C., Nelson, B. J., Shope, C. L., & Baffico, G. (2005b). Hydrogeochemistry and rare earth element behavior in a volcanically acidified watershed in Patagonia, Argentina. Chemical Geology, 222, 249–267.
Gimeno, M. J., Auqué, L. F., & Nordstrom, D. K. (2000). REE speciation in low-temperature acidic waters and the competitive effects of aluminum. Chemical Geology, 165, 167–180.
Gromet, L. P., Dymek, R. F., Haskin, L. A., & Korotev, R. L. (1984). The “North American shale composite”: its compilation, major and trace element characteristics. Geochimica et Cosmochimica Acta, 48, 2469–2482.
Hall, G. E. M., Vaive, J. E., & McConnell, J. W. (1995). Development and application of a sensitive and rapid analytical method to determine the rare-earth elements in surface waters. Chemical Geology, 120, 91–109.
Han, G., & Liu, C. Q. (2006). Dissolved rare earth elements in river waters draining karst terrains in Guizhou Province, China. Aquatic Geochemistry, 13, 95–107.
Haskin, L. A., Wildeman, T. R., & Haskin, M. A. (1968). An accurate procedure for the determination of the rare earths by neutron activation. Journal of Radioanalytical and Nuclear Chemistry, 1, 337–348.
Hu, Z., & Gao, S. (2008). Upper crustal abundances of trace elements: a revision and update. Chemical Geology, 253(3–4), 205–221.
Johannesson, K. H., & Lyons, W. B. (1995). Rare-earth element geochemistry of Colour Lake, an acidic freshwater lake on Axel Heiberg Island, Northwest Territories, Canada. Chemical Geology, 119, 209–223.
Kent, A. J. R., Jacobsen, B., Peate, D. W., Waight, T. E., & Baker, J. A. (2004). Isotope dilution MCICP-MS rare earth element analysis of geochemical reference materials NIST SRM 610, NIST SRM 612, NIST SRM 614, BHVO-2G, BHVO-2, BCR-2G, JB-2, WS-E, W-2, AGV-1 and AGV-2. Geostandards and Geoanalytical Research, 28(3), 417–429.
Knappe, A., Möller, P., Dulski, P., & Pekdeger, A. (2005). Positive gadolinium anomaly in surface water and ground water of the urban area Berlin, Germany. Chemie der Erde, 65, 167–189.
Kulaksiz, S., & Bau, M. (2011). Rare earth elements in the Rhine River, Germany: first case of anthropogenic lanthanum as a dissolved microcontaminant in the hydrosphere. Environment International, 37, 973–979.
Leybourne, M. I., Goodfellow, W. D., Boyle, D. R., & Hall, G. M. (2000). Rapid development of negative Ce anomalies in surface waters and contrasting REE patterns in groundwaters associated with Zn–Pb massive sulphide deposits. Applied Geochemistry, 15, 695–723.
Leybourne, M. I., & Johannesson, K. H. (2008). Rare earth elements (REE) and yttrium in stream waters, stream sediments, and Fe–Mn oxyhydroxides: fractionation, speciation, and controls over REE + Y patterns in the surface environment. Geochimica et Cosmochimica Acta, 72, 5962–5983.
Lichte, F. E., Meier, A. L., & Crock, J. G. (1987). Determination of the rare earth elements in geological materials by inductively coupled plasma mass spectrometry. Analytical Chemistry, 59, 1150–1157.
Markert, B., Piehler, H., Lieth, H., & Sugimae, A. (1989). Normalization and calculation of lanthanide element concentrations in environmental samples. Radiation and Environmental Biophysics, 28, 213–221.
McLennan, S. M. (1989). Rare earth elements in sedimentary rocks: influence of provenance and sedimentary processes. Reviews in Mineralogy and Geochemistry, 21, 169–200.
Merten, D., & Büchel, G. (2004). Determination of rare earth elements in acid mine drainage by inductively coupled plasma mass spectrometry. Microchimica Acta, 148, 163–170.
Michalik, A., & Migaszewski, Z. M. (2012). Stable sulfur and oxygen isotope ratios of the Świętokrzyski National Park spring waters generated by natural and anthropogenic factors (south-central Poland). Applied Geochemistry, 27, 1123–1132.
Migaszewski, Z. M., & Gałuszka, A. (2010). Xenotime from the Podwiśniówka mine pit, Holy Cross Mountains (South-Central Poland). Mineralogia, 41(1–2), 3–9.
Migaszewski, Z. M., Gałuszka, A., Hałas, S., Dołęgowska, S., Dąbek, J., & Starnawska, E. (2008). Geochemistry and stable sulfur and oxygen isotope ratios of the Podwiśniówka pit pond water generated by acid mine drainage (Holy Cross Mountains, south-central Poland). Applied Geochemistry, 23, 3620–3634.
Migaszewski, Z. M., Gałuszka, A., Michalik, A., Dołęgowska, S., Migaszewski, A., Hałas, S., & Trembaczowski, A. (2013). The use of stable sulfur, oxygen and hydrogen isotope ratios as geochemical tracers of sulfates in the Podwiśniówka acid drainage area (south-central Poland). Aquatic Geochemistry, 19, 261–280.
Migaszewski, Z. M., Gałuszka, A., Pasławski, P., & Starnawska, E. (2007a). An influence of pyrite oxidation on generation of unique acid pit water: a case study, Podwiśniówka quarry, Holy Cross Mountains (south-central Poland). Polish Journal of Environmental Studies, 16(3), 407–421.
Migaszewski, Z. M., Starnawska, E., & Gałuszka, A. (2007b). Gorceixite from the Upper Cambrian rocks of the Podwiśniówka mine pit, Holy Cross Mountains (south-central Poland). Mineralogia Polonica, 38(2), 171–184.
Ministry of Health. (2007). Regulation of the Minister of Health from March 29 of 2007 on the quality of water assigned to consumption by people. Law Gazette, 61, 417 (in Polish).
Nozaki, Y., Lerche, D., Alibo, D. S., & Tsutsumi, M. (2000). Dissolved indium and rare earth elements in three Japanese rivers and Tokyo Bay: evidence for anthropogenic Gd and In. Geochimica et Cosmochimica Acta, 64, 3975–3982.
Pourmand, A., Dauphas, N., & Ireland, T. J. (2012). A novel extraction chromatography and MC-ICP-MS technique for rapid analysis of REE, Sc and Y: Revising CI-chondrite and Post-Archean Australian Shale (PAAS) abundances. Chemical Geology, 291, 38–54.
Protano, G., & Riccobono, F. (2002). High contents of rare earth elements (REEs) in stream waters of a Cu–Pb–Zn mining area. Environmental Pollution, 117, 499–514.
Quinn, K. A., Byrne, R. H., & Schijf, J. (2006). Sorption of yttrium and rare earth elements by amorphous ferric hydroxide: influence of solution complexation with carbonate. Geochimica et Cosmochimica Acta, 70, 4151–4165.
Rasmussen, B., Buick, B., & Taylor, W. R. (1998). Removal of oceanic REE by authigenic precipitation of phosphatic minerals. Earth Planetary Science Letters, 164, 135–149.
Romero, F. M., Prol-Ledesma, R. M., Canet, C., Alvares, L. N., & Pérez-Vázquez, R. (2010). Acid drainage at the inactive Santa Lucia mine, western Cuba: natural attenuation of arsenic, barium and lead, and geochemical behavior of rare earth elements. Applied Geochemistry, 25, 716–727.
Steinmann, M., & Stille, P. (2008). Controls on transport and fractionation of the rare earth elements in stream water of a mixed basaltic–granitic catchment basin (Massif Central, France). Chemical Geology, 254, 1–18.
Sultan, K., & Shazili, N. A. (2009). Rare earth elements in tropical surface water, soil and sediments of the Terengganu River Basin, Malaysia. Journal of Rare Earths, 27(6), 1072–1078.
Tarvainen, T., & Salminen, R. (1997). FOREGS geochemical mapping field and laboratory manual. Geologian tut kimuskeskus, XX, 1–38.
Tricca, A., Stille, P., Steinmann, M., Kiefel, B., Samuel, J., & Eikenberg, J. (1999). Rare earth elements and Sr and Nd isotopic compositions of dissolved and suspended loads from small river systems in the Vosges mountains (France), the river Rhine and groundwater. Chemical Geology, 160(1–2), 139–158.
Verplanck, P. L., Antweiler, R. C., Nordstrom, D. K., & Taylor, H. E. (2001). Standard reference water samples for rare earth element determinations. Applied Geochemistry, 16, 231–244.
Verplanck, P. L., Nordstrom, D. K., Taylor, H. E., & Kimball, B. A. (2004). Rare earth element partitioning between hydrous ferric oxides and acid mine water during iron oxidation. Applied Geochemistry, 19, 1339–1354.
Wieser, M. E., & Schwieters, J. B. (2005). The development of multiple collector mass spectrometry for isotope ratio measurements. International Journal of Mass Spectrometry, 242(2–3), 97–115.
Worrall, F., & Pearson, D. G. (2001). Water–rock interaction in an acidic mine discharge as indicated by rare earth element patterns. Geochimica et Cosmochimica Acta, 65, 3027–3040.
Zhao, F., Cong, Z., Sun, H., & Ren, D. (2007). The geochemistry of rare earth elements (REE) in acid mine drainage from the Sitai coal mine, Shanxi Province, North China. International Journal of Coal Geology, 70, 184–192.
Żylińska, A., Szczepanik, Z., & Salwa, S. (2006). Cambrian of the Holy Cross Mountains, Poland: biostratigraphy of the Wiśniówka Hill succession. Acta Geologica Polonica, 56(4), 443–461.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Migaszewski, Z.M., Gałuszka, A. & Migaszewski, A. The study of rare earth elements in farmer's well waters of the Podwiśniówka acid mine drainage area (south-central Poland). Environ Monit Assess 186, 1609–1622 (2014). https://doi.org/10.1007/s10661-013-3478-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3478-7