Abstract
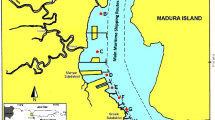
Nitroaromatic compounds are known to be hazardous to ecological and human health. To assess the status of nitroaromatic compounds contamination in the main rivers in the important industrial bases of the northeastern China, we collected water, suspended particulate matter (SPM) and sediment samples from 28 sites in the Daliao River watershed and analysed them for eight nitroaromatic compounds by gas chromatography. The total concentrations of eight nitrobenzenes in the water column including aqueous and SPM phases ranged from 740 to 15,828 ng L − 1, with a mean concentration of 3,460 ng L − 1. The total concentrations of eight nitrobenzenes in the sediment were 7.47 to 8,185.76 ng g − 1, with a mean concentration of 921.98 ng g − 1, and several times higher than those found from the Yellow River in China. 4-Nitrotoluene was the predominant contaminant in the water and sediment of the three rivers of the Daliao River watershed. 2,6-Dichloro-4-nitroaniline was generally dominant in the SPM. The levels of nitroaromatic compounds were different among different sites in the Daliao River watershed, mainly caused by the distribution of pollution sources. No obvious correlation was found between the total concentrations of eight nitrobenzenes concentrations and TOC or the slit-clay content of the sediments.
Similar content being viewed by others
References
Adkins, R. L. (1994). Nitrobenzene and nitrotoluene. In M. H. Grant (Ed.), Kirk Othmer encyclopedia of chemical technology, 4th edition. New York: Wiley.
Boyd, S. A., Sheng, G., Teppen, B. J., & Johnston, C. T. (2001). Mechanisms for the adsorption of substituted nitrobenzenes by smectite clays. Environmental Science & Technology, 35, 4227–4234.
Chen, M., Cui, L., Li, C. H., & Diao, G. W. (2009). Adsorption, desorption and condensation of nitrobenzene solution from active carbon: A comparison of two cyclodextrins and two surfactants. Journal of Hazardous Materials, 162, 23–28.
Chen, B. L., Huang, W. H., Mao, J. F., & Lv, S. F. (2008). Enhanced sorption of naphthalene and nitroaromatic compounds to bentonite by potassium and cetyltrimethylammonium cations. Journal of Hazardous Materials, 158, 116–123.
Colón, D., Weber, E. J., & Anderson, J. L. (2008). Effect of natural organic matter on the reduction of nitroaromatics by Fe(II) species. Environmental Science & Technology, 42, 6538–6543.
Du, Q. G. (2004). Stratagem study on sustainable development and environmental protection in Liaoning province (1st Edn., pp. 11–15). Beijing: Science (in Chinese).
Gao, J. J., Liu, L. H., Liu, X. R., Zhou, H. D., Wang, Z. J., & Huang, S. B. (2008). Concentration level and geographical distribution of nitrobenzene in Chinese surface waters. Journal of Environment Sciences, 20, 803–805.
Gatermann, R., Hühnerfuss, H., Rimkus, G., Wolf, M., & Franke, S. (1995). The distribution of Nitrobenzene and other nitroaromatic compounds in the north sea. Marine Pollution Bulletin, 30, 221–227.
Götz, R., Bauer, O. H., Firesel, R., & Roch, K. (1988). Organic trace compounds in the water of the river elbe near Hamburg. Chemosphere, 36, 2085–2101.
Guo, W., He, M. C., Yang, Z. F., Lin, C. Y., Quan, X. C., & Wang, H. Z. (2007). Distribution of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River watershed, China. Chemosphere, 68, 93–104.
Gustafson, K. E., & Dickhut, R. M. (1997). Distribution of polycyclic aromatic hydrocarbons tin Southern Chesapeake Bay surface water: Evaluation of three methods for determining freely dissolved water concentrations. Environmental Toxicology and Chemistry, 16, 452–461.
Hashimoto, A., Sakino, H., Yamagami, E., & Tateishi, S. (1980). Determination of dinitrotoluene isomers in sea water and industrial effluent by high-resolution electron-capture gas hromatography with a glass capillary column. Analyst, 1115, 787–793.
He, M. C., Sun, Y., Li, X. R., & Yang, Z. F. (2006). Distribution patterns of nitrobenzenes and polychlorinated biphenyls in water, suspended particulate matter and sediment from mid- and down-stream of the Yellow River (China). Chemosphere, 65, 365–374.
Kang, Y. H., Gong, Z. Y., Wang, Z. J., & Li, G. G. (2001). The study of VOCs in Guanting reservoir and Yongdinghe River. Acta Scienteae Circumstantiae, 21, 338–342 (in Chinese).
Kucklick, J. R., Sivertsen, S. K., Sanders, M., & Scott, G. I. (1992). Factors influencing polycyclic aromatic hydrocarbon distributions in South Carolina estuarine sediments. Journal of Experimental Marine Biology and Ecology, 213, 13–29.
Lang, P. Z., Long, F. S., Yuan, X., Ding, Y. Z., Wang, M. J., & Zhao, Y. H. (1993). The research on the toxic organic pollutants in water of middle stream of Songhua River (Shaokou-Songhua River Section). Advances in Environmental Science, 1, 47–55 (in Chinese).
Latifoglu, A., & Gurol, M. D. (2003). The effect of humic acids on nitrobenzene oxidation by ozonation and O3/UV processes. Water Research, 37, 1879–1889.
Li, H., Pereira, T. R., Teppen, B. J., Laird, D. A., Johnston, C. T., & Boyd, S. A. (2007). Ionic strength-induced formation of smectite quasicrystals enhances nitroaromatic compounds sorption. Environmental Science and Technology, 41, 1251–1256.
Li, H., Teppen, B. J., Johnston, C. J., & Boyd, S. A. (2004). Thermodynamics of nitroaromatic compound adsorption from water by smectite clay. Environmental Science and Technology, 38, 5433–5442.
Maskaoui, K., Zhou, J. L., Hong, H. S., & Zhang, Z. L. (2002). Contamination by polycyclic aromatic hydrocarbons in the Jiulong River Estuary and Western Xiamen Sea, China. Environmental Pollution, 118, 109–122.
Shen, X. Z., Liu, Z. C., Xie, S. M., & Guo, J. (2009). Degradation of nitrobenzene using titania photocatalyst co-doped with nitrogen and cerium under visible light illumination. Journal of Hazardous Materials, 162, 1193–1198.
Simpson, C. D., Mosi, A. A., Cullen, W. R., & Reimer, K. J. (1996). Composition and distribution of polycyclic aromatic hydrocarbons in surficial marine sediments from Kitimat Harbour, Canada. Science of the Total Environment, 181, 265–278.
Soil Science Society of China (1989). Conventional analysis methods of soil agricultural chemistry. Beijing: Science Press.
Tayade, R. J., Kulkarni, R. G., & Jasra, R. V. (2006). Photocatalytic degradation of aqueous nitrobenzene by nanocrystalline TiO2. Industrial and Engineering Chemistry Research, 45, 922–927.
U.S. EPA (1995). OPPT Chemical Fact Sheets, Nitrobenzene (CAS No. 98-95-3). EPA/749/F-95/015.
van de Meent, D., den Hollander, H. A., Pool, W. G., Vredenbregt, M. J., van Oers, H. A. M., de Greef, E., et al. (1986). Organic micropollutants in Dutch coastal waters. Water Science and Technology, 18, 73–81.
Vane, C. H., Harrison, I., & Kim, A. W. (2007). Polycyclic aromatic hydrocarbons (PAHs) and polychlorinated biphenyls (PCBs) in sediments from the Mersey Estuary, U.K. Science of the Total Environment, 374, 112–116.
Wang, H. Z., He, M. C., Lin, C. Y., Quan, X. C., Guo, W., & Yang, Z. F. (2007). Monitoring and assessment of persistent organochlorine residues in sediments from the Daliaohe River watershed, northeast of China. Environmental Monitoring and Assessment, 133, 231–242.
Wang, Z. J., Lv, Y. B., Wang, Y., & Ma, M. (2002). Assessing the ecological risk of substituted benzenes in Huaihe River, China. Acta Scientiae Circumstantiae, 22, 300–304 (in Chinese).
Wang, H., Yang, N. Y., Shen, Y. W., Wang, B., & Wang, L. X. (2003). Safety assessment on several organic pollutants of the Haihe River valley. Research Environment Sciences, 16, 35–36, 52 (in Chinese).
Wei, F. S. (1997). Guardline of analytical method for water and wastewater. Beijing: Chinese Environmental Science Press (in Chinese).
Yamagishi, T., Miyazaki, T., & Horii, S. (1981). Identification of musk xylene and musk ketone in fresh water fish collected from the Tama River Tokyo. Bulletin of Environmental Contamination and Toxicology, 26, 656–662.
Yan, L. B., & Baileyy, G. W. (2001). Sorption and abiotic redox transformation of nitrobenzene at the smectite–water interface. Journal of Colloid and Interface Science, 241, 142–153.
Yoshinori, N., & Tameo, O. (1995). Determination of nitrobenzenes in river water, sediment and fish samples by gas chromatography–mass spectrometry. Analytica Chimica Acta, 312, 45–55.
Yurawecz, M. P., & Puma, B. J. (1983). Nitro musks fragrances as potential contaminants in pesticide residue analysis. Journal Association of Official Analytical Chemists, 66, 241–247.
Zhao, L., Ma, J., Sun, Z. Z., & Zhai, X. D. (2008). Mechanism of influence of initial pH on the degradation of nitrobenzene in aqueous solution by Ceramic Honeycomb Catalytic Ozonation. Environmental Science & Technology, 42, 4002–4007
Zhao, X. K., Yang, G. P., & Gao, X. C. (2003). Studies on the sorption behaviors of nitrobenzene on marine sediments. Chemosphere, 52, 917–925.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Men, B., Wang, H., He, M. et al. Distribution patterns of nitroaromatic compounds in the water, suspended particle and sediment of the river in a long-term industrial zone (China). Environ Monit Assess 177, 515–526 (2011). https://doi.org/10.1007/s10661-010-1652-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-010-1652-8