Abstract
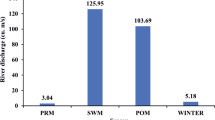
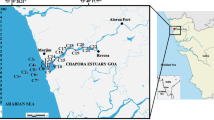
Studies on abundance and types of various pollution indicator bacterial populations from tropical estuaries are rare. This study was aimed to estimate current levels of pollution indicator as well as many groups of human pathogenic bacteria and their seasonal variations in different locations in Mandovi and Zuari Rivers in the central west coast of India. The sampling covered the estuarine and upstream regions of these rivers representing premonsoon (May 2005), monsoon (September 2006) and post-monsoon (November 2005). Both the abundance and types of autochthonous and allochthonous microbial populations in the near shore environments are affected by land drainages, domestic sewage outfalls and other discharges. The overall ranges (and their mean abundance; no. ml − 1) of the monitored groups of bacteria were: total coliforms: 0–29,047 (3,134 ml − 1); total streptococci: 3–14,597 (798); total vibrios: 13–42,275 (2,530); Escherichia coli: 0–1,333 (123); Vibrio cholerae: 0–3,012 (207); Salmonella spp: 0–1,646 (90); Streptococcus faecalis: 0–613 (88) and Aeromonas spp: 0–2,760 (205). In general, abundance of sewage pollution indicator bacteria such as total coliforms and total streptococci was lower than that reported from many other locations worldwide.
Similar content being viewed by others
References
Al-Harbi, A. H., & Uddin, N. (2003). Quantitative and qualitative studies on bacterial flora of hybrid tilapia (Qreochromis niloticus X O. aureus) cultured in earthen ponds in Saudi Arabia. Aquaculture and Research, 34, 43–48. doi:10.1046/j.1365-2109.2003.00791.x
American Public Health Association (APHA) (1980). Standard methods for the examination of water and wastewater (15th ed.). Washington, DC.
Brock, T., Madigan, M. T., Martinko, J. M., & Parker, J. (1994). Biology of microorganisms (7th edn.). New Jersey: Prentice hall.
Byrd, J. J., & Colwell, R. R. (1990). Maintenance of plasmids pBR322 and pUC8 in nonculturable Escherichia coli in the marine environment. Applied and Environmental Microbiology, 56, 2104–2107.
Cabelli, V. J. (1983). Health effects criteria for marine recreational waters. Washington, DC: Environmental Protection Agency
Colwell, R. R., Kaper, J., & Joseph, S. W. (1977). Vibrio cholerae, Vibrio parahaemolyticus, and other Vibrios: Occurrence and distribution in Chesapeake Bay. Science, 198, 394–396.
Colwell, R. R., Seidler, R. J., Kaper, J., Joseph, S. W., Garges, S., Lockman, H., et al. (1981). Occurrence of Vibrio cholerae serotype O1 in Maryland and Louisiana estuaries. Applied and Environmental Microbiology, 41, 555–558.
Darakas, E. (2001). A simple mathematical formula describing the survival kinetics of E. coli in natural waters. The International Journal of Environmental Studies, 58, 365–372. doi:10.1080/00207230108711338.
Dixon, B. (2005). Helicobacter from the seas? Studies around Italy’s coastline highlight potential dangers from human pathogens living in association with zooplankton. American Society for Microbiology, 71, 4–5.
Dufour, A. P. (1984). Bacterial indicators of recreational water quality. Canadian Journal of Public Health, 75, 49–56.
Fujioka, R. (2002). Microbial indicators of marine recreational water quality. In C. J. Hurst, R. L. Crawford, G. Knudsen, M. J. McIneney, L. D. Stetzenbach (Eds.), Manual of environmental microbiology (2nd ed., pp. 234–243). Washington DC: American Society for Microbiology Press.
Fujioka, R., Roll, B., & Byappanahalli, M. (1997). Appropriate recreational water quality standards for Hawaii and other tropical regions based on concentrations of Clostridium perfringens. In Proceedings of WEFTEC 97, 70th annual conference and exposition, surface water and ecology, (Vol. 4, pp. 405–411). Alexandria, VA: Water Environment Federation.
George, I., Petit, M., Theate, C., & Servais, P. (2001). Distribution of coliforms in the Seine River and Estuary (France) studied by rapid enzymatic methods and plate counts. Estuaries, 24, 94–102. doi:10.2307/1353012.
Hansen, B., & Bech, G. (1996). Bacteria associated with a marine planktonic copepod in culture. I. Bacterial genera in seawater, body surface, intestines and fecal pellets and succession during fecal pellet degradation. Journal of Plankton Research, 18(2), 257–273. doi:10.1093/plankt/18.2.257.
Hobbie, J. E., Daley, R. J., & Jasper, S. (1977). Use of nucleopore filters for counting bacteria by fluorescence microscopy. Applied and Environmental Microbiology, 33, 1225–1228.
Huq, A., West, P. A., Small, E. B., & Colwell, R. R. (1984). Influence of water temperature, salinity and pH on survival and growth of toxigenic Vibrio cholerae serovar O1 associated with live copepods in laboratory microcosms. Applied and Environmental Microbiology, 48, 420–424.
Islam, M. S., Siddika, A., Khan, M. N. H., Goldar, M. M., Sadique, M. A., Kabir, A. N. M. H., et al. (2001). Microbiological analysis of Tube-well water in a rural area of Bangladesh. Applied and Environmental Microbiology, 67(7), 3328–3330. doi:10.1128/AEM.67.7.3328-3330.2001.
Marchand, M. (1986). Ecological study of vibrios in Arcachon Bay, second international colloquium on marine bacteriology, Brest, 1–5, October 1984 (Vol. 3, pp. 483–489). Gerbam: CNRS. IFREMER: France.
McCarthy, S. A. (1996). Effect of temperature and salinity on survival of toxigenic Vibrio cholerae O1 in seawater. Microbial Ecology, 31, 167–175. doi:10.1007/BF00167862.
McCarthy, S. A., & Khambaty, F. M. (1994). International dissemination of epidemic Vibrio cholerae by cargo ship ballast and other nonpotable waters. Applied and Environmental Microbiology, 60, 2597–2601.
Ni, C. Z., & Lin, Y. S. (1986). The primary investigation of fecal Escherichia coli group in Hong Kong coastal waters. Marine Science Bulletin of Haiyang Tongbao, 5, 45–48.
Oliver, J. D., Hite, F., McDougald, D., Andon, N. L., & Simpson, L. M. (1995). Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Applied and Environmental Microbiology, 61, 2624–2630.
Patti, A. M., Paroli, E., Gabrieli, R., D’Angelo, A. M., De-Filippis, P., Villa, L., et al. (1987). Enteroviruses recovery from seawater: Statistical correlation with usual and chemical parameters. L’Igiene Moderna, 87, 226–243.
Piccolomini, R., Cellini, L., Allocati, N., Gentili, E., Sartorelli, M., & Di-Girolamo, A. (1987). Microbiological pollution of seawater. L’Igiene Moderna, 87, 543–552.
Ramaiah, N., & Chandramohan, D. (1993). Ecological and laboratory studies on the role of luminous bacteria and their luminescence in the coastal pollution surveillance. Marine Pollution Bulletin, 26, 190–201. doi:10.1016/0025-326X(93)90621-P.
Ramaiah, N., & De, J. (2003). Unusual rise in mercury resistant bacteria in coastal environments. Microbial Ecology, 45, 444–454. doi:10.1007/s00248-001-1068-7.
Ramaiah, N., Kenkre, V. D., & Verlecar, X. N. (2002b). Marine environmental pollution stress detection through direct viable counts of bacteria. Water Research, 36, 2383–2393. doi:10.1016/S0043-1354(01)00435-3.
Ramaiah, N., Kolhe, V., & Sadhasivan, A. (2004). Abundance of pollution indicator and pathogenic bacteria in Mumbai waters. Current Science, 87, 435–439.
Ramaiah, N., Ravel, J., Straube, W. L., Hill, R. T., & Colwell, R. R. (2002a). Entry of Vibrio harveyi and Vibrio fischeri into the viable and nonculturable state. Journal of Applied Microbiology, 93, 108–116. doi:10.1046/j.1365-2672.2002.01666.x.
Ruiz, G. M., Rawlings, T. K., Dobbs, F. C., Drake, L. A., Mullady, T., Huq, A., et al. (2000). Global spread of microorganisms by ships. Nature, 408, 49–50. doi:10.1038/35040695.
Sawkar, K., Pvethamony, P., Babu, M. T., Dias, C., Mesquita, A., Fernandes, B., et al. (2003). Measuring, modeling and grading the health of water bodies. In L. Noronha et al. (Eds.), Coastal tourism, environment, and sustainable local development (pp. 179–210). New Delhi: Tata Energy Research Institute.
Shankar, D., Vinayachandran, P. N., & Unnikrishnan, A. S. (2004). The monsoon currents in the north Indian Ocean. Progress in Oceanography, 52, 63–120. doi:10.1016/S0079-6611(02)00024-1.
Signoretto, C., Burlacchini, G., Lleo, M., Pruzzo, C., Zampini, M., Pane, L., et al. (2004). Adhesion of Enterococcus faecalis in the nonculturable state to plankton is the main mechanism responsible for persistence of this bacterium in both lake and seawater. Applied and Environmental Microbiology, 70(11), 6892–6896. doi:10.1128/AEM.70.11.6892-6896.2004.
Smith, J. J., Howington, J. P., & McFeters, G. A. (1994). Survival, physiological response, and recovery of enteric bacteria exposed to a polar environment. Applied and Environmental Microbiology, 60, 2977–2984.
Staley, J. T., & Konopka, A. (1985). Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Annual Review of Microbiology, 39, 321–346. doi:10.1146/annurev.mi.39.100185.001541.
Thom, S., Warhurst, D., & Drasar, B. S. (1992). Association of Vibrio cholerae with fresh water amoebae. Journal of Medical Microbiology, 36, 303–306.
Wait, D. A., & Sobsey, M. D. (2000). Comparative survival of enteric viruses and bacteria in Atlantic Ocean seawater. Water Science and Technology, 43, 139–142.
West, P. A., & Lee, J. V. (1982). Ecology of Vibrio species, including Vibrio cholerae, in natural waters of Kent, England. The Journal of Applied Bacteriology, 52, 435–448.
Xu, H. S., Roberts, N., Singleton, F. L., Attwell, R. W., Grimes, D. J., & Colwell, R. R. (1982). Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microbial Ecology, 8, 313–323. doi:10.1007/BF02010671.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagvenkar, G.S., Ramaiah, N. Abundance of sewage-pollution indicator and human pathogenic bacteria in a tropical estuarine complex. Environ Monit Assess 155, 245–256 (2009). https://doi.org/10.1007/s10661-008-0432-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-008-0432-1