Abstract
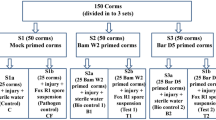
Populus davidiana × Populus bolleana (PdPap) root rot caused by Fusarium oxysporum is a major disease in China. Controlling this disease requires extensive use of chemicals. The use of plant endophytes, such as Bacillus, may be a suitable alternative to chemical agents. In this study, we isolated a strain of Bacillus amyloliquefaciens (AW3) by thermal stimulation and confrontation method from fleshy tap roots of Brassica rapa L. We then determined its inhibitory effect on the growth of F. oxysporum (Fox68) and how it induces the disease resistance of PdPap. The confrontation area and roots were visualized using an optical microscope and a scanning electron microscope, respectively, and mycelial cell malformation, swelling, and distortion observed. AW3 and F. oxysporum were inoculated in a variety of combinations that reduced the disease level. PdPap defense-related enzymes, such as PAL, PPO, SOD, and CAT, increased significantly. Besides, several genes associated with plant defense and hormonal signal transduction were highly expressed. Under the biological stress of Fox68, AW3 directly acted on the hyphae of Fox68, reducing the infection of PdPap by Fox68 and promoting PdPap growth. AW3 also induced the accumulation of defense-related enzymes/genes that conferred resistance. Therefore, AW3 could serve as a bio-control agent of wilt disease caused by F. oxysporum in PdPap.







Similar content being viewed by others
References
Ahmad, P., Sarwat, M., & Sharma, S. (2008). Reactive oxygen species, antioxidants and signaling in plants. Journal of Plant Biology, 51(3), 167–173.
Anderson, J. P., Schenk, P. M., Desmond, O. J., Maclean, D. J., Ebert, P. R., & Kazan, K. (2004). Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. The Plant Cell, 16(12), 3460–3479.
Addrah, M. E., Zhang, Y. Y., Zhang, J., Liu, L., Zhou, H. Y., Chen, W. D., & Zhao, J. (2019). Fungicide treatments to control seed-borne fungi of sunflower seeds. Pathogens, 9(1), 29.
Boukaew, S., Plubrukam, A., & Prasertsan, P. (2013). Effect of volatile substances from Streptomyces philanthi RM-1-138 on growth of rhizoctonia solani on rice leaf. BioControl, 58(4), 471–482.
Cameron, D. D., Neal, A. L., van Wees, S. C. M., & Ton, J. (2013). Mycorrhiza-induced resistance: More than the sum of its parts? Trends in Plant Science, 18(10), 539–545.
Coleman, J. J. (2016). The Fusarium solani species complex: Ubiquitous pathogens of agricultural importance. Molecular Plant Pathology, 17(2), 146–158.
Chen, L., Wang, X. H., Ma, Q. H., Bian, L. S., Liu, X., Xu, Y., Zhang, H. H., Shao, J. H., & Liu, Y. P. (2020). Bacillus velezensis CLA178-induced systemic resistance of rosa multiflora against crown gall disease. Frontiers in Microbiology. https://doi.org/10.3389/fmicb.2020.587667
Dimopoulou, A., Theologidis, I., Liebmann, B., Kalantidis, K., Vassilakos, N., & Skandalis, N. (2019). Bacillus amyloliquefaciens MBI600 differentially induces tomato defense signaling pathways depending on plant part and dose of application. Scientific Reports, 9, 19120.
Etesami, H., & Alikhani, H. A. (2016). Rhizosphere and endorhiza of oilseed rape (Brassica napus L.) plant harbor bacteria with multifaceted beneficial effects. Biological Control, 94, 11–24.
Etesami, H., & Maheshwari, D. K. (2018). Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicology and Environmental Safety, 156, 225–246.
Evenhuis, A., Verdam, B., Gerlagh, M., & Goossen-van de Geijn, H. M. (1995). Studies on major diseases of caraway (Carum carvi) in the Netherlands. Industrial Crops and Products, 4(1), 53–61.
Fan, W. H., & Dong, X. N. (2002). In vivo interaction between NPR1 and transcription factor TGA2 leads to salicylic acid-mediated gene activation in Arabidopsis. The Plant Cell, 14(6), 1377–1389.
Feng, N. X., Yu, J., Mo, C. H., Zhao, H. M., Li, Y. W., Wu, B. X., Cai, Q. Y., Li, H., Zhou, D. M., & Wong, M. H. (2018). Biodegradation of di-n-butyl phthalate (DBP) by a novel endophytes Bacillus megaterium strain YJB3. Science of the Total Environment, 616, 117–127.
Fravel, D. R. (2005). Commercialization and implementation of biocontrol. Annual Review of Phytopathology, 43, 337–359.
Gorash, A., Armoniene, R., & Kazan, K. (2021). Can effectoromics and loss-of-susceptibility be exploited for improving Fusarium head blight resistance in wheat? Crop Journal, 9, 1–16.
Hallmann, J., Quadt-Hallmann, A., Mahaffee, W. F., & Kloepper, J. W. (1997). Bacterial endophytes in agricultural crops. Canadian Journal of Microbiology, 43(10), 895–914.
Ivanova, P., Dzięgielewski, K., Drozd, M., Skorupska, M., Grabowska-Jadach, I., & Mariusz, P. (2021). Nanoparticles of chosen noble metals as reactive oxygen species scavengers. Nanotechnology. https://doi.org/10.1088/1361-6528/abc19f
Jiao, R., Munir, S., He, P. F., Yang, H. W., Wu, Y. X., Wang, J. W., He, P. B., Cai, Y. Z., Wang, G., & He, Y. Q. (2020). Biocontrol potential of the endophytes Bacillus amyloliquefaciens YN201732 against tobacco powdery mildew and its growth promotion. Biological Control. https://doi.org/10.1016/j.biocontrol.2019.104160
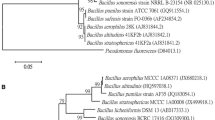
Kumar, S., Nei, M., Dudley, J., & Tamura, K. (2008). MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Briefings in Bioinformatics, 9(4), 299–306.
Lin, C. W., Chang, H. B., & Huang, H. J. (2005). Zinc induces mitogen-activated protein kinase activation mediated by reactive oxygen species in rice roots. Plant Physiology and Biochemistry, 43(10–11), 963–968.
Lian, L. L., Xie, L. Y., Zheng, L. P., & Lin, Q. Y. (2011). Induction of systemic resistance in tobacco against Tobacco mosaic virus by Bacillus spp. Biocontrol Science and Technology, 21(3), 281–292.
Lopes, R., Tsui, S., Gonçalves, P. J. R. O., & de Queiroz, M. V. (2018). A look into a multifunctional toolbox: Endophytes Bacillus species provide broad and underexploited benefits for plants. World Journal of Microbiology and Biotechnology, 34(7), 94.
Luna-Bulbarela, A., Tinoco-Valencia, R., Corzo, G., Kazuma, K., Konno, K., Galindo, E., & Serrano-Carreón, L. (2018). Effects of bacillomycin D homologues produced by Bacillus amyloliquefaciens 83 on growth and viability of Colletotrichum gloeosporioides at different physiological stages. Biological Control, 127, 145–154.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 25(4), 402–408.
Li, H. X., Xiao, Y., Cao, L. L., Yan, X., Li, C., Shi, H. Y., Wang, J. W., & Ye, Y. H. (2013). Cerebroside C increases tolerance to chilling injury and alters lipid composition in wheat roots. PLoS ONE, 8(9), e73380.
Nazari, M., & Smith, D. L. (2020). A PGPR-produced bacteriocin for sustainable agriculture: A review of Thuricin 17 characteristics and applications. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2020.00916
Ors, M., Randoux, B., Siah, A., Couleaud, G., Maumene, C., Sahmer, K., Reignault, P., Halama, P., & Selim, S. (2019). A plant nutrient- and microbial protein-based resistance inducer elicits wheat cultivar-dependent resistance against Zymoseptoria tritici. Phytopathology, 109(12), 2033–2045.
Peng, M. (1992). Peroxidase-generated hydrogen peroxide as a source of antifungal activity in vitro and on Tobacco leaf disks. Phytopathology, 82(6), 696–699.
Rabiey, M., Hailey, L. E., Roy, S. R., Grenz, K., Al-Zadjali, M. A. S., Barrett, G. A., & Jackson, R. W. (2019). Endophytes vs tree pathogens and pests: Can they be used as biological control agents to improve tree health? European Journal of Plant Pathology, 155(3), 711–729.
Ren, J. H., Li, H., Wang, Y. F., Ye, J. R., Yan, A. Q., & Wu, X. Q. (2013). Biocontrol potential of an endophytes Bacillus pumilus JK-SX001 against poplar canker. Biological Control, 67(3), 421–430.
Rooney, A. P., Price, N. P. J., Ehrhardt, C., Swezey, J. L., & Bannan, J. D. (2009). Phylogeny and molecular taxonomy of the Bacillus subtilis species complex and description of Bacillus subtilis subsp. inaquosorum subsp. nov. International Journal of Systematic and Evolutionary Microbiology, 59, 2429–2436.
Romero-Puertas, M. C., Rodriguez-Serrano, M., Corpas, F. J., Gomez, M., Del Rio, L. A., & Sandalio, L. M. (2004). Cadmium-induced subcellular accumulation of O2·- and H2O2 in pea leaves. Plant Cell and Environment, 27(9), 1122–1134.
Sánchez Hernández, M. E., Ruiz Dávila, A., Pérez de Algaba, A., Blanco López, M. A., & Trapero Casas, A. (1998). Occurrence and etiology of death of young olive trees in southern Spain. European Journal of Plant Pathology, 104, 347–357.
Selim, S., Martin-Laurent, F., Rouard, N., Gianinazzi, S., & van Tuinen, D. (2007). Impact of a new biopesticide produced by Paenibacillus sp. strain B2 on the genetic structure and density of soil bacterial communities. Pest Management Science, 63(3), 269–275.
Srikhong, P., Lertmongkonthum, K., Sowanpreecha, R., & Rerngsamran, P. (2018). Bacillus sp. strain M10 as a potential biocontrol agent protecting chili pepper and tomato fruits from anthracnose disease caused by Colletotrichum capsici. BioControl, 63(6), 833–842.
Schmitt, F. J., Renger, G., Friedrich, T., Kreslavski, V. D., Zharmukhamedov, S. K., Los, D. A., Kuznetsov, V. V., & Allakhverdiev, S. I. (2014). Reactive oxygen species: Re-evaluation of generation, monitoring and role in stress-signaling in phototrophic organisms. Biochimica Et Biophysica Acta Bioenergetics, 1837(6), 835–848.
Tashi-Oshnoei, F., Harighi, B., & Abdollahzadeh, J. (2017). Isolation and identification of endophytes bacteria with plant growth promoting and biocontrol potential from oak trees. Forest Pathology, 47(5), e12360.
Van Loon, L. C. (2007). Plant responses to plant growth-promoting rhizobacteria. European Journal of Plant Pathology, 119, 243–254.
Wang, J. F., Zhang, Y. Q., Li, Y., Wang, X. M., Nan, W. B., Hu, Y. F., Zhang, H., Zhao, C. Z., Wang, F., Li, P., Shi, H. Y., & Bi, Y. R. (2015). Endophytes microbes Bacillus sp. LZR216-regulated root development is dependent on polar auxin transport in Arabidopsis seedlings. Plant Cell Reports, 34(6), 1075–1087.
Whipps, J. M. (1997). Developments in the biological control of soil-borne plant pathogens. Advances in Botanical Research, 26, 1–134.
Wu, L. M., Huang, Z. Y., Li, X., Ma, L. M., Gu, Q., Wu, H. J., Liu, J., Borriss, R., Wu, Z., & Gao, X. W. (2018). Stomatal closure and SA-, JA/ET-Signaling pathways are essential for Bacillus amyloliquefaciens FZB42 to restrict leaf disease caused by Phytophthora nicotianae in Nicotiana benthamiana. Frontiers in Microbiology, 9, 847.
Weiss, A., Delproposto, J., & Giroux, C. N. (2014). High-throughput phenotypic profiling of gene-environment interactions by quantitative growth curve analysis in Saccharomyces cerevisiae. Analytical Biochemistry, 327(1), 23–34.
Xie, S., Vallet, M., Sun, C., Kunert, M., David, A., Zhang, X. C., Chen, B. H., Lu, X. M., Boland, W., & Shao, Y. Q. (2020). Biocontrol potential of a novel endophytes bacterium from Mulberry (Morus) tree. Frontiers in Bioengineering and Biotechnology, 7, 488.
Yaish, M. W., Antony, I., & Glick, B. R. (2015). Isolation and characterization of endophytes plant growth-promoting bacteria from date palm tree (Phoenix dactylifera L.) and their potential role in salinity tolerance. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology, 107(6), 1519–1532.
Yang, X. J., Chen, F. R., Gan, L., Du, Y. X., & Ruan, H. C. (2010). Effect of the endophytes Bacillus subtilis EBT1 isolated from banana on the growth and resistance to Fusarium wilt disease in banana. Acta Phytophylacica Sinica, 37(4), 300–306.
Yang, F., Li, W. S., Derbyshire, M., Larsen, M. R., Rudd, J. J., & Palmisano, G. (2015). Unraveling incompatibility between wheat and the fungal pathogen Zymoseptoria tritici through apoplastic proteomics. BMC Genomics, 16, 362.
Yamamoto, S., & Harayama, S. (1995). PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of pseudomonas putida strains. Applied and Environmental Microbiology, 61(10), 3768.
Yanez-Mendizabal, V., Zeriouh, H., Vinas, I., Torres, R., Usall, J., de Vicente, A., Perez-Garcia, A., & Teixido, N. (2012). Biological control of peach brown rot (Monilinia spp.) by Bacillus subtilis CPA-8 is based on production of fengycin-like lipopeptides. European Journal of Plant Pathology, 132(4), 609–619.
Zhang, C. X., Zhang, X. X., & Shen, S. H. (2014). Proteome analysis for antifungal effects of Bacillus subtilis KB-1122 on Magnaporthe grisea P131. World Journal of Microbiology and Biotechnology, 30(6), 1763–1774.
Acknowledgements
This work was supported by grants from the Fundamental Research Funds for the Centrol Universities (2572019AA05), Special Project for Double First-Class—Cultivation of Innovative Talents (000/41113102).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
All authors have read and approved the final manuscript. This manuscript is not under consideration by another journal and has not been previously published. This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zhang, P., Hao, H., Wang, L. et al. Endophytes Bacillus amyloliquefaciens AW3 (CGMCC1.16683) improves the growth of Populus davidiana × Populus bolleana (PdPap) and induces its resistance to wilt disease by Fusarium oxysporum Fox68 (CFCC86068). Eur J Plant Pathol 162, 1–17 (2022). https://doi.org/10.1007/s10658-021-02381-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-021-02381-x