Abstract
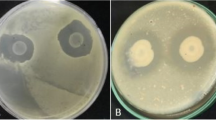
Black pod rot is the most significant factor limiting production of cocoa (Theobroma cacao) in Malaysia with average annual losses of above 30%. This work was carried out to isolate, characterize and screen bacterial endophytes from cocoa plants for their biological control activities. Their mechanisms of action as well as abilities to reduce black pod rot disease were also investigated. In total, 103 endophytic bacterial isolates were obtained from healthy cocoa tissues (leaves, branches and fruits) from seven states of Malaysia in 2016 and screened for their antagonism against P. palmivora in vitro. The best two isolates AS1 and AS2 with more than 80% inhibition of radial growth (PIRG) were selected for subsequent experiments. Sequence analysis of the 16S rRNA region indicated that these two isolates belonged to Pseudomonas aeruginosa (AS1) and Chryseobacterium proteolyticum (AS2). Bioactive volatile compounds were identified using gas chromatography-mass spectrometry (GCMS). Major compounds present in P. aeruginosa extract were identified as Eicosane (9.11%), Hexatriacontane (6.87%), Tetratetracontane (5.17%), trans-2-Decenoic acid (17.04%) and 1-Phenanthrenecarboxylic acid, 1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl) (3.60%). In C. proteolyticum extract, major compounds were identified as Eicosane (11.29%), Tetratetracontane (10.82%), Heneicosane (10.78%), Hexatriacontane (9.04%) and Phenol, 2,4-bis(1,1-dimethylethyl) (5.92%). Effectiveness of P. aeruginosa and C. proteolyticum in reducing black pod lesion was confirmed on detached cocoa pods with 100% inhibition for both isolates. These results indicated that these two bacterial isolates have potential to be used as bio-control agents against P. palmivora.





Similar content being viewed by others
References
Afsharmanesh, H., Ahmadzadeh, M., Javan-Nikkhah, M., & Behboudi, K. (2010). Characterization of the antagonistic activity of a new indigenous strain of Pseudomonas fluorescens isolated from onion rhizosphere. Journal of Plant Pathology, 187–194.
Ahmad Kamil, M. J. (2004). Antagonistic activities of epiphytic bacteria on black pod disease of cocoa. Master’s Thesis: The Senate of Universiti Putra, Malaysia.
Akrofi, A. Y., Amoako-Atta, I., Assuah, M., & Asare, E. K. (2015). Black pod disease on cacao (Theobroma cacao, L) in Ghana: Spread of Phytophthora megakarya and role of economic plants in the disease epidemiology. Crop Protection, 72, 66–75.
Akrofi, A. Y., Terlabie, J. L., Amoako-Attah, I., & Asare, E. K. (2017). Isolation and characterization of bacteria from different cacao progenies and their antagonistic activity against the black pod disease pathogen, Phytophthora palmivora. Journal of Plant Diseases and Protection, 124, 143–152.
Arnold, A. E., & Herre, E. A. (2003). Canopy cover and leaf age affect colonization by tropical fungal endophytes: Ecological pattern and process in Theobroma cacao (Malvaceae). Mycologia, 95, 388–398.
Arnold, A. E., Maynard, Z., Gilbert, G. S., Coley, P. D., & Kursar, T. A. (2000). Are tropical fungal endophytes hyperdiverse? Ecology Letters, 3, 267–274.
Bae, H., Sicher, R. C., Kim, M. S., Kim, S. H., Strem, M. D., Melnick, R. L., & Bailey, B. A. (2009). The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. Journal of Experimental Botany, 60, 3279–3295.
Bailey, B. A., Bae, H., Strem, M. D., Crozier, J., Thomas, S. E., Samuels, G. J., Vinyard, B. T., & Holmes, K. A. (2008). Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biocontrol potential in Theobroma cacao. Biological Control, 46, 24–35.
Belakhdar, G., Benjouad, A., & Abdennebi, E. H. (2015). Determination of some bioactive chemical constituents from Thesium humile Vahl. Journal of Materials and Environmental Science, 6, 2778–2783.
Belimov, A., Hontzeas, A., Safronova, N., Demchinskaya, V. I., Piluzza, S. V., Bulitta, G., & Glick, B. R. (2005). Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. czern.). Soil Biology and Biochemistry, 37, 241–250.
Borriss, R., Chen, X. H., Rueckert, C., Blom, J., Becker, A., Baumgarth, B., & Junge, H. (2011). Relationship of Bacillus amyloliquefaciens clades associated with strains DSM 7T and FZB42T: A proposal for Bacillus amyloliquefaciens subsp. amyloliquefaciens subsp. nov. and Bacillus amyloliquefaciens subsp. plantarum subsp. nov. based on complete genome sequence comparisons. International Journal of Systematic and Evolutionary Microbiology, 61, 1786–1801.
Cappuccino, J. G., & Sherman, N. (2008). Microbiology: A laboratory manual (Vol. 9). Pearson/Benjamin cummings.
Castillo, U. F., Strobel, G. A., Ford, E. J., Hess, W. M., Porter, H., Jensen, J. B., & Stevens, D. (2002). Munumbicins, wide-spectrum antibiotics produced by Streptomyces NRRL 30562, endophytic on Kennedia nigriscansa. Microbiology, 148, 2675–2685.
Chen, C., Bauske, E. M., Musson, G., Rodriguezkabana, R., & Kloepper, J. W. (1995). Biological control of Fusarium wilt on cotton by use of endophytic bacteria. Biological Control, 5, 83–91.
Compant, S., Duffy, B., Nowak, J., Clément, C., & Barka, E. A. (2005). Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Applied and Environmental Microbiology, 71, 4951–4959.
Dey, R. K. K. P., Pal, K. K., Bhatt, D. M., & Chauhan, S. M. (2004). Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiological Research, 159, 371–394.
Dunne, C., Delany, I., Fenton, A., & O'Gara, F. (1996). Mechanisms involved in biocontrol by microbial inoculants. Agronomie, 16, 721–729.
Fatima, Z., Saleemi, M., Zia, M., Sultan, T., Aslam, M., Rehman, R., & Chaudhary, M. F. (2009). Antifungal activity of plant growth-promoting rhizobacteria isolates against Rhizoctonia solani in wheat. African Journal of Biotechnology, 8, 219–225.
Galindo, J.J. (1992). Prospects for biological control of cacao. In: Keane, P.J., & putter, C. a. J. (Eds.). Cocoa pest and disease management in Southeast Asia and Australasia. Rome, FAO plant production and protection paper 112.
Gopalakrishnan, S., Pande, S., Sharma, M., Humayun, P., Kiran, B. K., Sandeep, D., & Rupela, O. (2011). Evaluation of actinomycete isolates obtained from herbal vermicompost for the biological control of Fusarium wilt of chickpea. Crop Protection, 30, 1070–1078.
Gummadi, S. N., & Panda, T. (2003). Purification and biochemical properties of microbial pectinases—A review. Process Biochemistry, 38, 987–996.
Hanada, R. E., de Jorge Souza, T., Pomella, A. W., Hebbar, K. P., Pereira, J. O., Ismaiel, A., & Samuels, G. J. (2008). Trichoderma martiale sp. nov., a new endophyte from sapwood of Theobroma cacao with a potential for biological control. mycological research, 112: 1335-1343.
Harman, G. E., Hayes, C. K., Lorito, M., Broadway, R. M., Di Pietro, A., Peterbauer, C., & Tronsmo, A. (1993). Chitinolytic enzymes of Trichoderma harzianum: Purification of chitobiosidase and endochitinase. Phytopathology, 83, 313–318.
Hashem, M., Alamri, S. A., Alrumman, S. A., & Moustafa, M. F. (2016). Suppression of phytopathogenic fungi by plant extract of some weeds and the possible mode of action. British Microbiology Research Journal, 15, 1–13.
Hassi, M., El Guendouzi, S., Haggoud, A., David, S., Ibnsouda, S., Houari, A., & Iraqui, M. (2012). Antimycobacterial activity of a Brevibacillus laterosporus strain isolated from a Moroccan soil. Brazilian Journal of Microbiology, 43, 1516–1522.
Herre, E. A., Mejía, L. C., Kyllo, D. A., Rojas, E., Maynard, Z., Butler, A., & Van Bael, S. A. (2007). Ecological implications of anti-pathogen effects of tropical fungal endophytes and mycorrhizae. Ecology, 88, 550–558.
Hsouna, A. B., Trigui, M., Mansour, R. B., Jarraya, R. M., Damak, M., & Jaoua, S. (2011). Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. International Journal of Food Microbiology, 148, 66–72.
Hu, X. F., Ying, F. X., He, Y. B., Gao, Y. Y., Chen, H. M., & Chen, J. S. (2008). Characterization of Pectobacterium carotovorum subsp. carotovorum causing soft-rot disease on Pinellia ternata in China. European Journal of Plant Pathology, 120, 305–310.
Husen, E. (2016). Screening of soil bacteria for plant growth promotion activities in vitro. Indonesian Journal of Agricultural Science, 4, 27–31.
Idan, A. A., Sijam, K., Kadir, J., Rashid, T. S., Awla, H. K., & Alsultan, W. (2017). Biological control of Pyricularia oryzae using antifungal compounds produced by Aspergillus niger. American Journal of Plant Sciences, 8, 2445–2460.
Intana, W., Chamswarng, C., Chantrapromma, K., Yenjit, P., Suwanno, C., & Sattasakulchai, S. (2008). Use of pentyl pyrone extracted from ultraviolet-induced mutant strain of Trichoderma harzianum for control leaf spot of Chinese-kale. Thai Journal of Agricultural Science, 41, 75–80.
Jayaraj, J., Parthasarathi, T., & Radhakrishnan, N. V. (2007). Characterization of a Pseudomonas fluorescens strain from tomato rhizosphere and its use for integrated management of tomato damping-off. Biological Control, 52, 683–702.
Joseph, B., Patra, R. R., & Lawrence, R. (2007). Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). International Journal of Plant Production, 1, 141–151.
Kamala, T., & Devi, S. I. (2012). Biocontrol properties of indigenous Trichoderma isolates from north-East India against Fusarium oxysporum and Rhizoctonia solani. African Journal of Biotechnology, 11, 8491–8499.
Karanja, E. N., Boga, H. I., Muigai, A. W., Wamunyokoli, F., Kinyua, J., & Nonoh, J. O. (2012, June). Growth characteristics and production of secondary metabolites from selected novel Streptomyces species isolated from selected Kenyan national parks. In Scientific Conference Proceedings.
Kararah, M. A., Barakat, F. M., Mikhail, M. S., & Fouly, H. M. (1985). Pathophysiology in garlic cloves inoculated with Bacillus subtilis, Bacillus pumilus and Erwinia carotovora. Egyptian Journal of Phytopathology, 17, 131–140.
Khamna, S., Yokata, A., & Lumyong, S. (2009). Actinomycetes isolated from medicinal plant rhizosphere soils: Diversity and screening of antifungal compounds, indole-3-acetic acid and siderophore production. World Journal of Biotechnology, 25, 649–655.
Kishore, G. K., Pande, S., & Podile, A. R. (2005). Phylloplane bacteria increase seedling emergence, growth and yield of field-grown groundnut (Arachis hypogaea L.). Letters in Applied Microbiology, 40, 260–268.
Kumar, U., & Dangar, T. K. (2013). Functional role of plant growth promoting Endo-and Rhizobacteria in major cereal crops. Kheti, 1, 37–40.
Lane, D.J. (1991). 16S/23S rRNA sequencing in: Stackebrandt, E. and good fellow, M., Ed. nucleic acid techniques in bacterial systematics. Pp. 115-175: NewYork, Wiley.
Li, P., Ma, L., Feng, Y. L., Mo, M. H., Yang, F. X., Dai, H. F., & Zhao, Y. X. (2012). Diversity and chemotaxis of soil bacteria with antifungal activity against Fusarium wilt of banana. Journal of Industrial Microbiology & Biotechnology, 39, 1495–1505.
Liu, Y., Guo, J., Li, L., Asem, M. D., Zhang, Y., Mohamad, O. A., & Li, W. (2017). Endophytic bacteria associated with endangered plant Ferula sinkiangensis KM Shen in an arid land: Diversity and plant growth-promoting traits. Journal of Arid Land, 9, 432–445.
Lodewyckx, C., Vangronsveld, J., Porteous, F., Moore, E. R., Taghavi, S., Mezgeay, M., & der Lelie, D. V. (2002). Endophytic bacteria and their potential applications. Critical Reviews in Plant Sciences, 21, 583–606.
Macagnan, D., Romeiro, R. D. S., de Souza, J. T., & Pomella, A. W. (2006). Isolation of actinomycetes and endospore-forming bacteria from the cacao pod surface and their antagonistic activity against the witches’ broom and black pod pathogens. Phytoparasitica, 34, 122–132.
Maurhofer, M., Keel, C., Haas, D., & Défago, G. (1995). Influence of plant species on disease suppression by Pseudomonas fluorescens strain CHAO with enhanced antibiotic production. Plant Pathology, 44, 40–50.
Minorsky, P. V. (2008). On the inside. Plant Physiology 146: 323–324 Neilands J. B., Nakamura K. (1991). In G. Winkelmann (Ed.), CRC handbook of microbial iron chelates (pp. 1–14). Florida: CRC Press.
Mishra, M., Kumar, U., Mishra, P. K., & Prakash, V. (2010). Efficiency of plant growth promoting rhizobacteria for the enhancement of Cicer arietinum L. growth and germination under salinity. Advances in Biological Research, 4, 92–96.
Montealegre, J. R., Reyes, R., Pérez, L. M., Herrera, R., Silva, P., & Besoain, X. (2003). Selection of bioantagonistic bacteria to be used in biological control of Rhizoctonia solani in tomato. Electronic Journal of Biotechnology, 6, 115–127.
Nautiyal, C. S. (1999). An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiology Letters, 170, 265–270.
Nimnoi, P., Pongsilp, N., & Lumyong, S. (2010). Endophytic actinomycetes isolated from Aquilaria crassna Pierre ex Lec and screening of plant growth promoters production. World Journal of Microbiology and Biotechnology, 26, 193–203.
Padwick, G.W. (1956). Losses caused by plant diseases in the colonies. Volume 1, Phyto pathological papers. Kew, England, commonwealth mycological institute. (no. 632 P33).
Paramageetham, C., & Prasada Babu, G. (2012). Antagonistic Activity of Fluorescent Pseudomonads against a Polyphagous Soil Born Plant Pathogen-Sclerotium rolfsii., 1, 436.
Podile, A. R., & Kishore, G. K. (2006). Plant growth-promoting rhizobacteria. In plant-associated bacteria (pp. 195-230). Springer Netherlands.
Prabukumar, S., Rajkuberan, C., Ravindran, K., & Sivaramakrishnan, S. (2015). Isolation and characterization of endophytic fungi from medicinal plant Crescentia cujete L. and their antibacterial, antioxidant and anticancer properties. International Journal of Pharmacy and Pharmaceutical Sciences, 7, 316–321.
Rassouli, M. H., Khavazi, K., Rahimian, H., Malakouti, M. J., & Asadi-Rahmani, H. (2005). An evaluation of the potential of indigenous Fluorescent Pseudomonds of wheat rhizosphere for producing siderophore. Iran Journal of Soil and Water Science, 20, 133–143.
Reiter, B., Pfeifer, U., Schwab, H., & Sessitsch, A. (2002). Response of endophytic bacterial communities in potato plants to infection with Erwinia carotovora subsp. atroseptica. Applied and Environmental Microbiology, 68, 2261–2268.
Ross, I. L., Alami, Y., Harvey, P. R., Achouak, W., & Ryder, M. H. (2000). Genetic diversity and biological control activity of novel species of closely related pseudomonads isolated from wheat field soils in South Australia. Applied and Environmental Microbiology, 66, 1609–1616.
Sahaf, B. Z., Moharramipour, S., & Meshkatalsadat, M. H. (2007). Chemical constituents and fumigant toxicity of essential oil from Carum copticum against two stored product beetles. Insect Sci., 14, 213–218.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual. 2rd edition, cold Spring Harbor laboratory press, cold spring harbour, New York. USA. pp., 9, 31–9.57.
Samuel, S., & Muthukkaruppan, S. M. (2011). Characterization of plant growth promoting rhizobacteria and fungi associated with rice, mangrove and effluent contaminated soil. Current Botany, 2, 22–25.
Sessitsch, A., Reiter, B., & Berg, G. (2004). Endophytic bacterial communities of field-grown potato plants and their plant-growth-promoting and antagonistic abilities. Canadian Journal of Microbiology, 50, 239–249.
Sheoran, N., Nadakkakath, A. V., Munjal, V., Kundu, A., Subaharan, K., Venugopal, V., & Kumar, A. (2015). Genetic analysis of plant endophytic Pseudomonas putida BP25 and chemo-profiling of its antimicrobial volatile organic compounds. Microbiological Research, 173, 66–78.
Siddiqui, I. A., Shaukat, S. S., Sheikh, I. H., & Khan, A. (2006). Role of cyanide production by Pseudomonas fluorescens CHA0 in the suppression of root-knot nematode, Meloidogyne javanica in tomato. World Journal of Microbiology and Biotechnology, 22, 641–650.
Silva, P. D., & Nahas, E. (2002). Bacterial diversity in soil in response to different plans, phosphate fertilizers and liming. Brazilian Journal of Microbiology, 33, 304–310.
Singh, Y., Singh, J., & Pandey, A. K. (2013). Molecular markers in diagnosis and management of fungal pathogens: A review. International Journal of Advanced Biotechnology and Research, 4, 180–188.
Sivakamasundari, R., & Usharani, G. (2012). Studies on the influence of Pseudomonas fluorescens and chemicals on the biocontrol sheath blight incidence in rice. International Journal of Pharmaceutical & Biological Archives, 3, 973–977.
Smibert, R. M., & Krieg, N. R. (1994). Phenotypic characterization. In methods for general and molecular bacteriology. American Society for Microbiology, 611-651.
Sturz, A. V., Christie, B. R., & Nowak, J. (2000). Bacterial endophytes: Potential role in developing sustainable systems of crop production. Critical Reviews in Plant Sciences, 19, 1–30.
Swadling, I. R., & Jeffries, P. (1998). Antagonistic properties of two bacterial biocontrol agents of grey mould disease. Biocontrol Science and Technology, 8, 439–448.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Tokala, R. K., Strap, J. L., Jung, C. M., Crawford, D. L., Salove, M. H., Deobald, L. A., & Morra, M. J. (2002). Novel plant-microbe rhizosphere interaction involving Streptomyces lydicus WYEC108 and the pea plant (Pisum sativum). Applied and Environmental Microbiology, 68, 2161–2171.
Wahyudi, A. T., & Astuti, R. I. (2011). Screening of Pseudomonas sp. isolated from rhizosphere of soybean plant as plant growth promoter and biocontrol agent. American Journal of Agricultural and Biological Science.
Williams, G. E., & Asher, M. J. C. (1996). Selection of rhizobacteria for the control of Pythium ultimum and Aphanomyces cochlioides on sugar-beet seedlings. Crop Protection, 15, 479–486.
Yasmin, F., Othman, R., Sijam, K., & Saad, M. S. (2010). Characterization of beneficial properties of plant growth-promoting rhizobacteria isolated from sweet potato rhizosphere. African Journal of Microbiology Research, 3, 815–821.
Zhang, X., Li, B., Wang, Y., Guo, Q., Lu, X., Li, S., & Ma, P. (2013). Lipopeptides, a novel protein, and volatile compounds contribute to the antifungal activity of the biocontrol agent Bacillus atrophaeus CAB-1. Applied Microbiology and Biotechnology, 97, 9525–9534.
Acknowledgments
I would like to express my heartfelt and deep appreciation to my late supervisor Associate. Prof. Dr. Jugah Kadir for his valuable assistance in this study which have remarkable contributed to fulfill this research with less difficulty.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animals rights
No human and/or animal participants were involved in this research.
Informed consent
All authors consent to this submission.
Electronic supplementary material
ESM 1
(DOCX 3297 kb)
Rights and permissions
About this article
Cite this article
Alsultan, W., Vadamalai, G., Khairulmazmi, A. et al. Isolation, identification and characterization of endophytic bacteria antagonistic to Phytophthora palmivora causing black pod of cocoa in Malaysia. Eur J Plant Pathol 155, 1077–1091 (2019). https://doi.org/10.1007/s10658-019-01834-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01834-8