Abstract
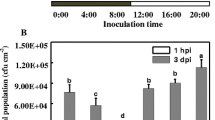
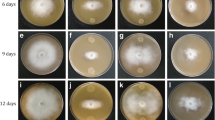
Increasing evidence suggests that the interactions between plant and pathogen are influenced by light perception of both organisms. In our previous study, green light decreased disease severity in tomato plants in response to Pseudomonas cichoriiJBC1 (PcJBC1) infection via induction of disease resistance-related genes. However, the influence of green light on gene expression and metabolism of PcJBC1 has not been explored. In this study, we cultured PcJBC1 in hrp-inducing minimal media (MM) under green light and dark condition, and analyzed the expression of genes, which are important in pathogenicity/virulence and epiphytic survival by real-time PCR (qPCR). Although no significant changes were observed in the expression of type 3 secretion system (T3SS)- and flagellar-related genes in response to green light, genes for the production of phytotoxic lipopeptides and siderophores were significantly reduced by green light. In addition, the phytotoxic lipopeptide and siderophore production in the culture supernatant was consistent with the results of gene expression. Furthermore, we compared the whole transcriptomes of PcJBC1 grown in MM under green light and dark condition. Among 243 differentially expressed genes (>2-fold change), the photoreceptor genes, bacteriophytochrome (bphP) and heme-oxygenase (bphO) were significantly up-regulated, whereas genes involved in the type 1 secretion system (T1SS), type 6 secretion system (T6SS), phytotoxic lipopeptides, and iron acquisition were profoundly repressed under green light. Corresponding to qPCR analysis, the RNA-seq results also showed no significant alteration in the T3SS- and flagellar-related genes. Overall, our results suggest that green light perceived by PcJBC1 plays a key role in diverse physiological responses that might affect this pathogen’s epiphytic survival. The receptor and signaling network involved in green light perception should be identified in further investigation.






Similar content being viewed by others
References
Bechtold, U., Karpinski, S., & Mullineaux, P. M. (2005). The influence of the light environment and photosynthesis on oxidative signaling responses in plant–biotrophic pathogen interactions. Plant Cell & Environment., 28, 1046–1055.
Bender, C. L., Alarcón-Chaidez, F., & Gross, D. C. (1999). Pseudomonas syringae phytotoxins: Mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiology and Molecular Biology Review., 63, 266–292.
Bonomi, H. R., Posadas, D. M., Paris, G., CarricaMdel, C., Frederickson, M., Pietrasanta, L. I., Bogomolni, R. A., Zorreguieta, A., & Goldbaum, F. A. (2012). Light regulates attachment, exopolysaccharide production, and nodulation in Rhizobium leguminosarum through a LOV-histidine kinase photoreceptor. Proceedings of the National Academy of Sciences of the United States of America., 109, 12135–12140.
Bonomi, H. R., Toum, L., Sycz, G., Sieira, R., Toscani, A. M., Gudesblat, G. E., Leskow, F. C., Goldbaum, F. A., Vojnov, A. A., & Malamud, F. (2016). Xanthomonas campestris attenuates virulence by sensing light through a bacteriophytochrome photoreceptor. EMBO Reports., 17, 1565–1577.
Bultreys, A., Gheysen, I., Wathelet, B., Schäfer, M., & Budzikiewicz, H. (2004). The pyoverdins of Pseudomonas syringae and Pseudomonas cichorii. ZeitschriftfürNaturforschung, 59, 613–618.
Cheng, D. D., Liu, M. J., Sun, X. B., Zhao, M., Chow, W. S., Sun, G. Y., Zhang, Z. S., & Hu, Y. B. (2016). Light suppresses bacterial population through the accumulation of hydrogen peroxide in tobacco leaves infected with Pseudomonas syringae pv. tabaci. Frontiers in Plant Science, 7, 512.
Choquer, M., Lee, M. H., Bau, H. J., & Chung, K. R. (2007). Deletion of a MFS transporter-like gene in Cercospora nicotianae reduces cercosporin toxin accumulation and fungal virulence. FEBS Letters., 581, 489–494.
Claret, L., Miquel, S., Vieille, N., Ryjenkov, D. A., Gomelsky, M., & Darfeuille-Michaud, A. (2007). The flagellar sigma factor FliA regulates adhesion and invasion of Crohn disease-associated Escherichia coli via a cyclic dimeric GMP-dependent pathway. Journal of Biological Chemistry., 282, 33275–33283.
Coulthurst, S. (2013). The type VI secretion system - a widespread and versatile cell targeting system. Research in Microbiology., 164, 640–654.
Delprato, M. L., Krapp, A. R., & Carrillo, N. (2015). Green light to plant responses to pathogens: The role of chloroplast light-dependent signaling in biotic stress. Photochemistry and Photobiology., 91, 1004–1011.
Franza, T., & Expert, D. (2013). Role of iron homeostasis in the virulence of phytopathogenic bacteria: An ‘à la carte’ menu: Fe homeostasis and bacterial phytopathogenicity. Molecular Plant Pathology., 14, 429–438.
Gomelsky, M., & Hoff, W. D. (2011). Light helps bacteria make important lifestyle decisions. Trends in Microbiology., 19, 441–448.
Green, E. R., & Mecsas, J. (2016). Bacterial secretion systems – An overview. Microbiology Spectrum., 4. doi:10.1128/microbiolspec.VMBF-0012-2015.
Hikichi, Y., Wali, U. M., Ohnishi, K., & Kiba, A. (2013). Mechanism of disease development caused by a multihost plant bacterium, Pseudomonas cichorii, and its virulence diversity. Journal of General Plant Pathology., 79, 379–389.
Hojo, H., Koyanagi, M., Tanaka, M., Kajihara, S., Ohnishi, K., Kiba, A., & Hikichi, Y. (2008). The hrp genes of Pseudomonas cichorii are essential for pathogenicity on eggplant but not on lettuce. Microbiolology., 154, 2920–2928.
Huang, C. J., Pauwelyn, E., Ongena, M., Debois, D., Leclère, V., Jacques, P., Bleyaert, P., & Höfte, M. (2015). Characterization of cichopeptins, new phytotoxic cyclic lipodepsipeptides produced by Pseudomonas cichorii SF1-54 and their role in bacterial midrib rot disease of lettuce. Molecular Plant-Microbe Interactions., 28, 1009–1022.
Hung, N. B., Ramkumar, G., & Lee, Y. H. (2014). An effector gene hopA1 influences on virulence, host specificity, and lifestyles of Pseudomonas cichorii JBC1. Research in Microbiology, 165, 620–629.
Huynh, T. V., Dahlbeck, D., & Staskawicz, B. J. (1989). Bacterial blight of soybean: Regulation of a pathogen gene determining host cultivar specificity. Science, 245, 1374–1377.
Jenewein, S., Barry Holland, I., & Schmitt, L. (2009). Type I bacterial secretion systems. In K. Wooldridge (Ed.), Bacterial secreted proteins: Secretory mechanisms and role in pathogenesis (pp. 45–65). Caister Academic Press: Norwich.
Karpinski, S., Gabrys, H., Mateo, A., Karpinska, B., & Mullineaux, P. M. (2003). Light perception in plant disease defence signalling. Current Opinion in Plant Biology., 4, 390–396.
Kiba, A., Takata, O., Ohnishi, K., & Hikichi, Y. (2006). Comparative analysis of induction pattern of programmed cell death and defense-related responses during hypersensitive cell death and development of bacterial necrotic leaf spots in eggplant. Planta, 224, 981–994.
Kraiselburd, I., Alet, A. I., Tondo, M. L., Petrocelli, S., Daurelio, L. D., Monzon, J., Ruiz, O. A., Losi, A., & Orellano, E. G. (2012). A LOV protein modulates the physiological attributes of Xanthomonas axonopodis pv. citri relevant for host plant colonization. PLoS ONE, 7, e38226.
Kraiselburd, I., Moyano, L., Carrau, A., Tano, J., & Orellano, E. G. (2017). Bacterial photosensory proteins and their role in plant–pathogen interactions. Photochemistry and Photobiology., 93, 666–674.
Lee, Y. H., Kolade, O. O., Nomura, K., Arvidson, D. N., & He, S. Y. (2005). Use of dominant-negative HrpA mutants to dissect Hrp pilus assembly and type III secretion in Pseudomonas syringaepv. tomato. Journal of Biological Chemistry., 280, 21409–21417.
Liao, H. L., & Chung, K. R. (2008). Genetic dissection defines the roles of elsinochromephytotoxin for fungal pathogenesis and conidiation of the citrus pathogen Elsinoe fawcettii. Molecular Plant-Microbe Interactions., 21, 469–479.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real time quantitative PCR and the 2-∆∆CT method. Methods, 25, 402–408.
Miethke, M., & Marahiel, M. A. (2007). Siderophore-based iron acquisition and pathogen control. Microbiology and Molecular Biology Review., 71, 413–451.
Mussi, M. A., Gaddy, J. A., Cabruja, M., Arivett, B. A., Viale, A. M., Rasia, R., & Actis, L. A. (2010). The opportunistic human pathogen Acinetobacter baumannii senses and responds to light. Journal of Bacteriology., 192, 6336–6345.
Nagendran, R., & Lee, Y. H. (2015). Green and red light reduces the disease severity by Pseudomonas cichorii JBC1 in tomato plants via upregulation of defense-related gene expression. Phytopathology, 105, 412–418.
Nomura, K., Mecey, C., Lee, Y. N., Imboden, L. A., Chang, J. H., & He, S. Y. (2011). Effector-triggered immunity blocks pathogen degradation of an immunity-associated vesicle traffic regulator in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America., 108, 10774–10779.
Oberpichler, I., Rosen, R., Rasouly, A., Vugman, M., Ron, E. Z., & Lamparter, T. (2008). Light affects motility and infectivity of Agrobacterium tumefaciens. Environmental Microbiology., 10, 2020–2029.
Pauwelyn, E., Huang, C. J., Ongena, M., Leclère, V., Jacques, P., Bleyaert, P., Budzikiewicz, H., Schäfer, M., & Höfte, M. (2013). New linear lipopeptides produced by Pseudomonas cichorii SF1-54 are involved in virulence, swarming motility, and biofilm formation. Molecular Plant-Microbe Interactions., 26, 585–598.
Ramkumar, G., Yu, S., & Lee, Y. H. (2013). Influence of light qualities on antifungal lipopeptide synthesis in Bacillus amyloliquefaciens JBC36. European Journal of Plant Pathology, 137, 243–248.
Ramkumar, G., Lee, S. W., Weon, H.-Y., Kim, B.-Y., & Lee, Y. H. (2015). First report on the whole genome sequence of Pseudomonas cichorii strain JBC1 and comparison with other Pseudomonas species. Plant Patholology., 64, 63–70.
Ricci, A., Dramis, L., Shah, R., Gartner, W., & Losi, A. (2015). Visualizing the relevance of bacterial blue- and red-light receptors during plant-pathogen interaction. Environmental Microbiology Reports., 7, 795–802.
Río-Álvarez, I., Rodríguez-Herva, J. J., Martínez, P. M., González-Melendi, P., García-Casado, G., Rodríguez-Palenzuela, P., & López-Solanilla, E. (2014). Light regulates motility, attachment and virulence in the plant pathogen Pseudomonas syringae pv. tomato DC3000. Environmental Microbiology., 16, 2072–2085.
Roden, L. C., & Ingle, R. A. (2009). Lights, rhythms, infection: The role of light and the circadian clock in determining the outcome of plant-pathogen interactions. The Plant Cell, 21, 2546–2552.
Scholz-Schroeder, B. K., Hutchison, M. L., Grgurina, I., & Gross, D. C. (2001). The contribution of syringopeptin and syringomycin to virulence of Pseudomonas syringae pv. syringae strain B301D on the basis of sypA and syrB1 biosynthesis mutant analysis. Molecular Plant-Microbe Interactions., 14, 336–348.
Schwarz, S., Hood, R. D., & Mougous, J. D. (2010). What is type VI secretion doing in all those bugs? Trends in Microbiology., 18, 531–537.
Schwyn, B., & Neilands, J. B. (1987). Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry, 160, 47–56.
Taguchi, F., Suzuki, T., Inagaki, Y., Toyoda, K., Shiraishi, T., & Ichinose, Y. (2010). The siderophore pyoverdine of Pseudomonas syringae pv. tabaci 6605 is an intrinsic virulence factor in host tobacco infection. Journal of Bacteriology., 192, 117–126.
Terauchi, K., Montgomery, B. L., Grossman, A. R., Lagarias, J. C., & Kehoe, D. M. (2004). RcaE is a complementary chromatic adaptation photoreceptor required for green and red light responsiveness. Molecular Microbiology., 51, 567–577.
van der Horst, M. A., Key, J., & Hellingwerf, K. J. (2007). Photosensing in chemotrophic, non-phototrophic bacteria: Let there be light sensing too. Trends in Microbiology., 15, 554–562.
Wagner, K., Besemer, K., Burns, N. R., Battin, T. J., & Bengtsson, M. M. (2015). Light availability affects stream biofilm bacterial community composition and function, but not diversity. Environmental Microbiology, 17, 5036–5047.
Wali, U. M., Maenaka, M., Mori, Y., Ueno, D., Kai, K., Ohnishi, K., Kiba, A., Hayashi, H., & Hikichi, Y. (2015). Implication of limited iron acquisition by Pseudomonas cichorii strain SPC9018 in its reduced virulence on eggplant. Journal of General Plant Pathology., 81, 136–141.
Wu, L., McGrane, R. S., & Beattie, G. A. (2013). Light regulation of swarming motility in Pseudomonas syringae integrates signaling pathways mediated by a bacteriophytochrome and a LOV protein. MBio, 4, e00334–e00313.
Yu, S. M., & Lee, Y. H. (2013). Effect of light quality on Bacillus amyloliquefaciens JBC36 and its biocontrol efficacy. Biological Control, 64, 203–210.
Yu, S. M., Ramkumar, G., & Lee, Y. H. (2013). Light quality influences the virulence and physiological responses of Colletotrichum acutatum causing anthracnose in pepper plants. Journal of Applied Microbiology., 115, 509–516.
Zeier, J., Pink, B., Mueller, M. J., & Berger, S. (2004). Light conditions influence specific defense responses in incompatible plant–pathogen interactions: Uncoupling systemic resistance from salicylic acid and PR-1 accumulation. Planta, 219, 673–683.
Acknowledgements
This research was supported by the Basic Science Research Program (2014R1A1A4A01003957 and 2017R1A2B2002221) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests
Electronic supplementary material
ESM 1
(DOCX 111 kb)
Rights and permissions
About this article
Cite this article
Rajalingam, N., Lee, Y.H. Effects of green light on the gene expression and virulence of the plant pathogen Pseudomonas cichorii JBC1. Eur J Plant Pathol 150, 223–236 (2018). https://doi.org/10.1007/s10658-017-1270-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1270-1