Abstract
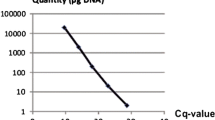
Apple proliferation (AP), caused by ‘Candidatus Phytoplasma mali’, is an economically important disease affecting many apple-growing areas in Europe. A new TaqMan real-time PCR assay was established for absolute quantification of ‘Ca. P. mali’ by using a single-copy gene of the host plant as a reference, which is amplified with the pathogen DNA in a single-tube reaction. Normalised estimates of phytoplasma concentration are ultimately expressed as the number of phytoplasma cells per host plant cell. The assay was used to monitor the ‘Ca. P. mali’ titre over the course of two growing seasons in roots and branches of symptomatic and asymptomatic but AP-positive apple trees. All 252 root samples from symptomatic and asymptomatic trees tested positive, with an average number of 59.8 ± 5.68 (standard error) and 55.1 ± 9.83 ‘Ca. P. mali’ per host cell, respectively. From the 378 shoot samples analysed, 81% of the symptomatic and only 20% of the asymptomatic samples were AP-positive with an average number of 9.4 ± 1.04 and 0.7 ± 0.13 ‘Ca. P. mali’ per host cell, respectively. This strengthens evidence that not the pathogen occurrence alone but the presence of a certain quantity of ‘Ca. P. mali’ in the aerial tree sections is involved in symptom expression. In addition, pronounced seasonality of the phytoplasma concentration was found, not only in branches, but also for the first time in roots of symptomatic and asymptomatic apple trees. Highest phytoplasma levels in roots were detected from December to May.



Similar content being viewed by others
References
Abdel-Farid, B., Jahangir, M., van den Hondel, C. A. M. J. J., Kim, H. K., Choi, Y. H., & Verpoorte, R. (2009). Fungal infection-induced metabolites in Brassica rapa. Plant Science, 176, 608–615.
Baric, S., & Dalla Via, J. (2004). A new approach to apple proliferation detection: a highly sensitive real-time PCR assay. Journal of Microbiological Methods, 57, 135–145.
Baric, S., Kerschbamer, C., & Dalla Via, J. (2006). TaqMan real-time PCR versus four conventional PCR assays for detection of apple proliferation phytoplasma. Plant Molecular Biology Reporter, 24, 169–184.
Baric, S., Kerschbamer, C., & Dalla Via, J. (2007). Detection of latent apple proliferation infection in two differently aged apple orchards in South Tyrol (northern Italy). Bulletin of Insectology, 60, 265–266.
Baric, S., Kerschbamer, C., Vigl, J., & Dalla Via, J. (2008a). Translocation of apple proliferation phytoplasma via natural root grafts—a case study. European Journal of Plant Pathology, 121, 207–211.
Baric, S., Monschein, S., Hofer, M., Grill, D., & Dalla Via, J. (2008b). Comparability of genotyping data obtained by different procedures—an interlaboratory survey. Journal of Horticultural Science and Biotechnology, 83, 183–190.
Bertaccini, A. (2007). Phytoplasmas: diversity, taxonomy, and epidemiology. Frontiers in Bioscience, 12, 673–689.
Bisognin, C., Schneider, B., Salm, H., Grando, M. S., Jarausch, W., Moll, E., et al. (2008). Apple proliferation resistance in apomictic rootstocks and its relationship to phytoplasma concentration and simple sequence repeat genotypes. Phytopathology, 98, 153–158.
Brunner, K., Paris, M. P. K., Paolino, G., Burstmayr, H., Lemmens, M., Berthiller, F., et al. (2009). A reference-gene-based quantitative PCR method as a tool to determine Fusarium resistance in wheat. Analytical and Bioanalytical Chemistry, 395, 1385–1394.
Carraro, L., Ermacora, P., Loi, N., & Osler, R. (2004). The recovery phenomenon in apple proliferation-infected apple trees. Journal of Plant Pathology, 86, 141–146.
Christensen, N. M., Nicolaisen, M., Hansen, M., & Schulz, A. (2004). Distribution of phytoplasmas in infected plants as revealed by real-time PCR and bioimaging. Molecular Plant-Microbe Interactions, 17, 1175–1184.
Ciccotti, A., Bianchedi, P. L., Bragagna, P., Deromedi, M., Filippi, M., Forno, F., et al. (2008). Natural and experimental transmission of Candidatus Phytoplasma mali by root bridges. Acta Horticulturae, 781, 459–464.
Costa, F., Stella, S., Van de Weg, W. E., Guerra, W., Cecchinel, M., Dalla Via, J., et al. (2005). Role of the genes Md-ACO1 and Md-ACS1 in ethylene production and shelf life of apple (Malus domestica Borkh). Euphytica, 141, 181–190.
Evert, R. F. (1963). The cambium and seasonal development of the phloem in Pyrus malus. American Journal of Botany, 50, 149–159.
Frisinghelli, C., Delaiti, L., Grando, M. S., Forti, D., & Vindimian, M. E. (2000). Cacopsylla costalis (Flor 1861), as a vector of apple proliferation in Trentino. Journal of Phytopathology, 148, 425–431.
Hogenhout, S. A., Oshima, K., Ammar, E. D., Kakizawa, S., Kingdom, H. N., & Namba, S. (2008). Phytoplasmas: bacteria that manipulate plants and insects. Molecular Plant Pathology, 9, 403–423.
Jarausch, W., Peccerella, T., Schwind, N., & Krczal, G. (2004). Establishment of a quantitative real-time PCR assay for the quantification of apple proliferation phytoplasmas in plants and insects. Acta Horticulturae, 657, 415–420.
Kaminska, M., Gabryszewska, E., Korbin, M., & Rudzinska-Langwald, A. (2002). Phytoplasma detection in some ornamental plants propagated in vitro. Acta Horticulturae, 568, 237–245.
Kartte, S., & Seemüller, E. (1988). Variable response within the genus Malus to the apple proliferation disease. Journal of Plant Diseases and Protection, 95, 25–34.
Lee, I. M., Davis, R. E., & Gundersen-Rindal, D. E. (2000). Phytoplasma: phytopathogenic mollicutes. Annual Review of Microbiology, 54, 221–255.
Lloyd, A. D., Mellerowicz, E. J., Chow, C. H., Riding, R. T., & Little, C. H. A. (1994). Fluctuations in ribosomal RNA gene content and nucleolar activity in the cambial region of Abies balsamea shoots during reactivation. American Journal of Botany, 81, 1384–1390.
Loi, N., Ermacora, P., Carraro, L., Osler, R., & Chen, T. A. (2002). Production of monoclonal antibodies against apple proliferation phytoplasma and their use in serological detection. European Journal of Plant Pathology, 108, 81–86.
Musetti, R., di Toppi, L. S., Ermacora, P., & Favali, M. A. (2004). Recovery in apple trees infected with the apple proliferation phytoplasma: An ultrastructural and biochemical study. Phytopathology, 94, 203–208.
Oshima, K., Kakizawa, S., Arashida, R., Ishii, Y., Hoshi, A., Hayashi, Y., et al. (2007). Presence of two glycolytic gene clusters in a severe pathogenic line of Candidatus Phytoplasma asteris. Molecular Plant Pathology, 8, 481–489.
Pedrazzoli, F., Ciccotti, A., Bianchedi, P., Salvadori, A., & Zorer, R. (2008). Seasonal colonisation behaviour of ‘Candidatus Phytoplasma mali’ in apple trees in Trentino. Acta Horticulturae, 781, 483–489.
Rogers, S. O., & Bendich, A. J. (1987). Ribosomal RNA genes in plants: variability in copy number and in the intergenic spacer. Plant Molecular Biology, 9, 509–520.
Saracco, P., Bosco, D., Veratti, F., & Marzachì, C. (2006). Quantification over time of chrysanthemum yellows phytoplasma (16Sr-I) in leaves and roots of the host plant Chrysanthemum carinatum (Schousboe) following inoculation with its insect vector. Physiological and Molecular Plant Pathology, 67, 212–219.
Schaper, U., & Seemüller, E. (1982). Conditions of the phloem and the persistence of mycoplasmalike organisms associated with apple proliferation and pear decline. Phytopathology, 72, 736–742.
Schaper, U., & Seemüller, E. (1984). Recolonization of the stem of apple proliferation and pear decline-diseased trees by the causal organisms in spring. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz, 91, 608–613.
Schmid, G. (1975). Prolonged observations on spread and behaviour of proliferation disease in apple orchards. Acta Horticulturae, 44, 183–192.
Schneider, B., & Seemüller, E. (1994). Presence of two sets of ribosomal genes in phytopathogenic Mollicutes. Applied and Environmental Microbiology, 60, 3409–3412.
Schneider, B., Seemüller, E., Smart, C. D., & Kirkpatrick, B. C. (1995). Phylogenetic classification of plant pathogenic mycoplasma-like organisms or phytoplasmas. In S. Razin & J. G. Tully (Eds.), Molecular and diagnostic procedures in mycoplasmology, Vol. 1 (pp. 369–380). San Diego: Academic Press.
Sears, B. B., Klomparens, K. L., Wood, J. I., & Schewe, G. (1997). Effect of altered levels of oxygen and carbon dioxide on phytoplasma abundance in Oenothera leaf tip cultures. Physiological and Molecular Plant Pathology, 50, 275–287.
Seemüller, E., & Schneider, B. (2004). ‘Candidatus Phytoplasma mali’, ‘Candidatus Phytoplasma pyri’ and ‘Candidatus Phytoplasma prunorum’, the causal agents of apple proliferation, pear decline and European stone fruit yellows, respectively. International Journal of Systematic and Evolutionary Microbiology, 54, 1217–1226.
Seemüller, E., & Schneider, B. (2007). Differences in virulence and genomic features of strains of ‘Candidatus Phytoplasma mali’, the apple proliferation agent. Phytopathology, 97, 964–970.
Seemüller, E., Kunze, L., & Schaper, U. (1984a). Colonization behavior of MLO, and symptom expression of proliferation-diseased apple trees and decline-diseased pear trees over a period of several years. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz, 91, 525–532.
Seemüller, E., Schaper, U., & Zimbelmann, F. (1984b). Seasonal variation in the colonization patterns of mycoplasmalike organisms associated with apple proliferation and pear decline. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz, 91, 371–382.
Sivaci, A. (2006). Seasonal changes of total carbohydrate contents in three varieties of apple (Malus sylvestris Miller) stem cuttings. Scientia Horticulturae, 109, 234–237.
Tedeschi, R., & Alma, A. (2004). Transmission of apple proliferation phytoplasma by Cacopsylla melanoneura (Homoptera: Psyllidae). Journal of Economic Entomology, 97, 8–13.
Tedeschi, R., & Alma, A. (2006). Fieberiella florii (Homoptera: Auchenorrhyncha) as a vector of “Candidatus Phytoplasma mali”. Plant Disease, 90, 284–290.
Torres, E., Bertolini, E., Cambra, M., Montón, C., & Martín, M. P. (2005). Real-time PCR for simultaneous and quantitative detection of quarantine phytoplasmas from apple proliferation (16SrX) group. Molecular and Cellular Probes, 19, 334–340.
Tromp, J. (1983). Nutrient reserves in roots of fruit trees, in particular carbohydrates and nitrogen. Plant and Soil, 71, 401–413.
Valsesia, G., Gobbin, D., Patocchi, A., Vecchione, A., Pertot, I., & Gessler, C. (2005). Development of a high-throughput method for quantification of Plasmopara viticola DNA on grape leaves by means of quantitative real-time polymerase chain reaction. Phytopathology, 95, 672–678.
Varma, A., Padh, H., & Shrivastava, N. (2007). Plant genomic DNA isolation: an art or a science. Biotechnology Journal, 2, 386–392.
Acknowledgments
The authors are grateful to T. Alber for making his orchard available for sample collection, to M. Wolf for providing data on symptom monitoring and to M. Falk for help with statistical analyses. This work was funded by the Autonomous Province of Bozen/Bolzano, Italy. The South Tyrolean Fruit Growers’ Co-operatives, in particularly VOG and VIP, are acknowledged for co-financing the Strategic Project on Apple Proliferation—APPL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baric, S., Berger, J., Cainelli, C. et al. Seasonal colonisation of apple trees by ‘Candidatus Phytoplasma mali’ revealed by a new quantitative TaqMan real-time PCR approach. Eur J Plant Pathol 129, 455–467 (2011). https://doi.org/10.1007/s10658-010-9706-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-010-9706-x