Abstract
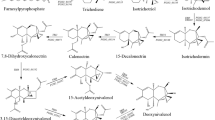
This review describes the naturally occurring mechanisms in cereals that lead to a reduction of Fusarium trichothecene mycotoxin accumulation in grains. A reduction in mycotoxin contamination in grains could also limit fungal infection, as trichothecenes have been reported to act as virulence factors. The mechanisms explaining the low toxin accumulation trait, generally referred to as type V resistance to Fusarium, can be subdivided into two classes. Class 1 includes mechanisms by which the plants chemically transform the trichothecenes, leading to their degradation or detoxification. Among the detoxification strategies, glycosylation of trichothecenes is a natural process already reported in wheat. According to the structure and the toxicity of trichothecenes, two other detoxification processes, acetylation and de-epoxidation, can be expressed, at least in transgenic plants. Class 2 comprises mechanisms that lead to reduced mycotoxin accumulation by inhibition of their biosynthesis through the action of plant endogenous compounds. These include both grain constitutive compounds and compounds induced in response to pathogen infection. There are already many compounds with antioxidant properties, like phenolic compounds, peptides or carotenoids, and with prooxidant properties, like hydrogen peroxide or linoleic acid-derived hydroperoxides, that have been described as ‘modulators’ of mycotoxin biosynthesis. This review addresses for the first time different studies reporting specific in vitro effects of such compounds on the biosynthesis of Fusarium mycotoxins. A better understanding of the natural processes limiting accumulation of trichothecenes in the plant will open the way to the development of novel breeding varieties with reduced ‘mycotoxin risk’.
Similar content being viewed by others
Abbreviations
- 3-ADON:
-
3-acetyl-4-deoxynivalenol
- 15-ADON:
-
15-acetyl-4-deoxynivalenol
- 4-ABOA:
-
4-acetyl-benzoxazolin-2-one
- 9S-HPODE:
-
9S-hydroperoxide
- 13S-HPODE:
-
13S-hydroperoxide
- DON:
-
deoxynivalenol
- FHB:
-
Fusarium head blight
- FX:
-
fusarenone X
- LC:
-
liquid chromatography
- LOX:
-
lipoxygenase
- MS:
-
mass spectrometry
- NIV:
-
nivalenol
- QTL:
-
quantitative trait loci
- TCT B:
-
trichothecene B
- UDP glycosyltransferase:
-
uridine diphosphate glycosyltransferase
References
Abdel-Aal, E. S. M., Young, J. C., Rabalski, I., Hucl, P., & Fregeau-Reid, J. (2007). Identification and quantification of seed carotenoids in selected wheat species. Journal of Agricultural and Food Chemistry, 55, 787–794.
Apel, K., Bohlmann, H., & Reimann-Philipp, U. (1990). Leaf thionins, a novel class of putative defence factors. Physiologia Plantarum, 80, 315–321.
Assabgui, R. A., Reid, L. M., Hamilton, R. I., & Arnason, J. T. (1993). Correlation of kernel (E)-ferulic acid content of maize with resistance to Fusarium graminearum. Phytopathology, 83, 949–953.
Atkinson, H. A. C., & Miller, K. (1984). Inhibitory effect of deoxynivalenol, 3-acetyldeoxynivalenol and zearalenone on induction of rat and human lymphocyte proliferation. Toxicology Letters, 23, 215–221.
Aziz, N. H., Farag, S. E., Mousa, L. A. A., & Abo-Zaid, M. A. (1998). Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios, 93, 43–54.
Bakan, B., Bily, A. C., Melcion, D., Cahagnier, B., Regnault-Roger, C., Philogene, B. J. R., et al. (2003). Possible role of plant phenolics in the production of trichothecenes by Fusarium graminearum strains on different fractions of maize kernels. Journal of Agricultural and Food Chemistry, 51, 2826–2831.
Beekrum, S., Govinden, R., Padayachee, T., & Odhav, B. (2003). Naturally occurring phenols: a detoxification strategy for fumonisin B1. Food Additives and Contaminants, 20, 490–493.
Bell, A. A. (1981). Biochemical mechanisms of disease resistance. Annual Review of Plant Physiology, 32, 21–81.
Bennett, J. W., & Klich, M. (2003). Mycotoxins. Clinical Microbiology Reviews, 16, 497–516.
Berthiller, F., Dall’Asta, C., Schuhmacher, R., Lemmens, M., Adam, G., & Krska, R. (2005). Masked mycotoxins: Determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 53, 3421–3425.
Bily, A. (2003). Rôle et importance des déhydrodimères d’acide férulique et autres phénylpropanoïdes dans les mécanismes de résistance de Zea mays L. à Fusarium graminearum Schwabe. Doctoral Thesis, Pau University, France.
Bily, A. C., Reid, L. M., Taylor, J. H., Johnston, D., Malouin, C., Burt, A. J., et al. (2003). Dehydrodimers of ferulic acid in maize grain pericarp and aleurone: resistance factors to Fusarium graminearum. Phytopathology, 93, 712–719.
Binder, E. M. (2007). Managing the risk of mycotoxins in modern feed production. Animal Feed Science and Technology, 133, 149–166.
Binder, E. M., Heidler, D., Schatzmayr, G., Thimm, N., Fuchs, E., Schuh, M., et al. (2000). Mycotoxins and phycotoxins in perspective at the turn of the millennium. (Paper presented at the 10th International IUPAC Symposium on Mycotoxins and Phycotoxins, Guarujá, Brazil).
Bottalico, A., & Perrone, G. (2002). Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. European Journal of Plant Pathology, 108, 611–624.
Bruins, M. B. M., Karsai, I., Schepers, J., & Snijders, C. H. A. (1993). Phytotoxicity of deoxynivalenol to wheat tissue with regard to in vitro selection for Fusarium head blight resistance. Plant Science, 94, 195–206.
Burow, G. B., Nesbitt, T. C., Dunlap, J., & Keller, N. P. (1997). Seed lipoxygenase products modulate Aspergillus mycotoxin biosynthesis. Molecular Plant-Microbe Interactions, 10, 380–387.
Castoria, R., de Luca, C., Fabbri, A. A., Passi, S., & Fanelli, C. (1989). By-products of lipoperoxidation and aflatoxin production. Journal of Toxicology, 8, 349–360.
Champeil, A., Dore, T., & Fourbet, J. F. (2004). Fusarium head blight: epidemiological origin of the effects of cultural practices on head blight attacks and the production of mycotoxins by Fusarium in wheat grains. Plant Science, 166, 1389–1415.
Chen, Z. Y., Brown, R. L., Lax, A. R., Guo, B. Z., Cleveland, T. E., & Russin, J. S. (1998). Resistance to Aspergillus flavus in corn kernels is associated with a 14- kDa protein. Phytopathology, 88, 276–281.
Chen, Z. Y., Brown, R. L., Rajasekaran, K., Damann, K. E., & Cleveland, T. E. (2006). Identification of a maize kernel pathogenesis-related protein and evidence for its involvement in resistance to Aspergillus flavus infection and aflatoxin production. Phytopathology, 96, 87–95.
Chipley, J. R., & Uraih, N. (1980). Inhibition of Aspergillus growth and aflatoxin release by derivatives of benzoic acid. Applied and Environmental Microbiology, 40, 352–357.
Coleman, J. O. D., Blake-Kalff, M. M. A., & Davies, T. G. E. (1997). Detoxification of xenobiotics by plants: chemical modification and vacuolar compartmentation. Trends in Plant Science, 2, 144–151.
Dall’Asta, C., Berthiller, F., Schuhmacher, R., Adam, G., Lemmens, M., & Krska, R. (2005). DON-glycosides: characterisation of synthesis products and screening for their occurrence in DON-treated wheat samples. Mycotoxin Research, 21, 123–127.
Desjardins, A. E., Hohn, T. M., & McCormick, S. P. (1993). Trichothecene biosynthesis in Fusarium species: chemistry, genetics and significance. Microbiological Reviews, 57, 595–604.
Desjardins, A. E., Plattner, R. D., & Spencer, G. F. (1988). Inhibition of trichothecene toxin biosynthesis by naturally occurring shikimate aromatics. Phytochemistry, 27, 767–771.
Doohan, F. M., Mentewab, A., & Nicholson, P. (2000). Antifungal activity toward Fusarium culmorum in soluble wheat extracts. Phytopathology, 90, 666–671.
Duvick, J. P., Rood, T., Rao, A. G., & Marshak, D. R. (1992). Purification and characterization of a novel antimicrobial peptide from maize (Zea mays L.) kernels. Journal of Biological Chemistry, 267, 18814–18820.
Edwards, S. G. (2004). Influence of agricultural practices on Fusarium infection of cereals and subsequent contamination of grain by trichothecene mycotoxins. Toxicology Letters, 153, 29–35.
Egorov, T. A., Odintsova, T. I., Pukhalsky, V. A., & Grishin, E. V. (2005). Diversity of wheat anti-microbial peptides. Peptides, 26, 2064–2073.
El-Banna, A. A. (1987). Stability of citrinin and deoxynivalenol during germination process of barley. Mycotoxin Research, 3, 37–41.
Eriksen, G. S. (2003). Metabolism and toxicity of trichothecenes. Doctoral Thesis, Uppsala University, Sweden.
Eriksen, G. S., Pettersson, H., & Lundh, T. (2004). Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de-epoxy metabolites. Food and Chemical Toxicology, 42, 619–624.
Eudes, F., Comeau, A., Rioux, S., & Collin, J. (2000). Phytotoxicity of eight mycotoxins associated with the fusariosis of wheat spikelets. Canadian Journal of Plant Pathology, 22, 286–292.
Fabbri, A. A., Fanelli, C., Panfili, G., Passi, S., & Fasella, P. (1983). Lipoperoxidation and aflatoxin biosynthesis by Aspergillus parasiticus and A. flavus. Journal of General Microbiology, 129, 3447–3452.
Fanelli, C., & Fabbri, A. A. (1989). Relationship between lipids and aflatoxin biosynthesis. Mycopathologia, 107, 115–120.
Fanelli, C., Fabbri, A. A., Panfili, G., Castoria, R., Luca, C. D., & Passi, S. (1989). Aflatoxin congener biosynthesis induced by lipoperoxidation. Experimental Mycology, 13, 61–68.
Favre L., Verdal-Bonnin, M. N., Pinson-Gadais, L., Roumet, P., Barreau, C., & Richard- Forget, F. (2004). Does biochemical composition of durum wheat kernels influence the trichothecenes B (TCT B) contamination levels? (Paper presented at the 2nd International Symposium on Fusarium Head Blight, Orlando, Florida, USA).
Friend, J. (1981). Plant phenolics, lignification and plant disease. Progress in Phytochemistry, 7, 197–261.
Fritig, B., Heitz, T., & Legrand, M. (1998). Antimicrobial proteins in induced plant defense. Current Opinion in Immunology, 10, 16–22.
Fuchs, E., Binder, E. M., Heidler, D., & Krska, R. (2002). Structural characterization of metabolites after the microbial degradation of type A trichothecenes by the bacterial strain BBSH 797. Food Additives and Contaminants, 19, 379–386.
Fujita, M., & Yoshizawa, T. (1990). Metabolism of deoxynivalenol, a trichothecene mycotoxin, in sweet potato root tissues. Journal of the Food Hygienic Society of Japan, 31, 474–478.
Gardner, H. W. (1991). Recent investigations into the lipoxygenase pathways of plants. Biochimica and Biophysica Acta, 1084, 221–239.
Gareis, M., Bauer, J., Thiem, J., Plank, G., Grabley, S., & Gedek, B. (1990). Cleavage of zearalenone-glycoside, a “masked” mycotoxin during digestion in swine. Journal of Veterinary Medicine, 37, 236–240.
Garvey, G. S., McCormick, S. P., & Rayment, I. (2007). Structural and functional characterization of the TRI101 trichothecene 3-O-acetyltransferase from Fusarium sporotrichioides and Fusarium graminearum; kinetic insights to combating Fusarium head blight. Journal of Biological Chemistry. DOI 10.1074/m705752200.
Goodrich-Tanrikulu, M., Mahoney, N. E., & Rodriguez, S. B. (1995). The plant growth regulator methyl jasmonate inhibits aflatoxin production by Aspergillus flavus. Microbiology, 141, 2831–2837.
Guiraud, P., Steiman, R., Seigle-Murandi, F., & Benoit-Guyod, J. L. (1995). Comparison of the toxicity of various lignin-related phenolic compounds toward selected fungi perfecti and fungi imperfecti. Ecotoxicology and Environmental Safety, 32, 29–33.
Hazel, C. M., & Patel, S. (2004). Influence of processing on trichothecene levels. Toxicology Letters, 153, 51–59.
Hentschel, V., Kranl, K., Hollmann, J., Lindhauer, M. G., Bohm, V., & Bitsch, R. (2002). Spectrophotometric determination of yellow pigment content and evaluation of carotenoids by high-performance liquid chromatography in durum wheat grain. Journal of Agricultural and Food Chemistry, 50, 6663–6668.
Hua, S.-S. T., Grosjean, O.-K., & Baker, J. L. (1999). Inhibition of aflatoxin biosynthesis by phenolic compounds. Letters in Applied Microbiology, 29, 289–291.
Huang, Z., White, D. G., & Payne, G. A. (1997). Corn seed proteins inhibitory to Aspergillus flavus and aflatoxin biosynthesis. Phytopathology, 87, 622–627.
Huynh, Q. K., Borgmeyer, J. R., & Zobel, J. F. (1992b). Isolation and characterization of a 22 kDa protein with antifungal properties from maize seeds. Biochemical and Biophysical Research Communications, 182, 1–5.
Huynh, Q. K., Hironaka, C. M., Levine, E. B., Smith, C. E., Borgmeyer, J. R., & Shah, D. M. (1992a). Antifungal proteins from plants. Purification, molecular cloning, and antifungal properties of chitinases from maize seed. Journal of Biological Chemistry, 267, 6635–6640.
Jones, P., & Vogt, T. (2001). Glycosyltransferases in secondary plant metabolism: tranquilizers and stimulant controllers. Planta, 213, 164–174.
Kachroo, A., He, Z. H., Patkar, R., Zhu, Q., Zhong, J., Li, D., et al. (2003). Induction of H2O2 in transgenic rice leads to cell death and enhanced resistance to both bacterial and fungal pathogens. Transgenic Research, 12, 577–586.
Kimura, M., Kaneko, I., Komiyama, M., Takatsuki, A., Koshino, H., Yoneyama, K., et al. (1998). Trichothecene 3-O-acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins - Cloning and characterization of Tri101. Journal of Biological Chemistry, 273, 1654–1661.
Kimura, M., Takahashi-Ando, N., Nishiuchi, T., Ohsato, S., Tokai, T., Ochiai, N., et al. (2006). Molecular biology and biotechnology for reduction of Fusarium mycotoxin contamination. Pesticide Biochemistry and Physiology, 86, 117–123.
Konopka, I., Czaplicki, S., & Rotkiewicz, D. (2006). Differences in content and composition of free lipids and carotenoids in flour of spring and winter wheat cultivated in Poland. Food Chemistry, 95, 290–300.
Lee, S. E., Campbell, B. C., Molyneux, R. J., Hasegawa, S., & Lee, H. S. (2001). Inhibitory effects of naturally occurring compounds on aflatoxin B1 biotransformation. Journal of Agricultural and Food Chemistry, 49, 5171–5177.
Lemmens, M., Scholz, U., Berthiller, F., Dall’Asta, C., Koutnik, A., Schuhmacher, R., et al. (2005). The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Molecular Plant-Microbe Interactions, 18, 1318–1324.
Lempereur, I., Rouau, X., & Abecassis, J. (1997). Genetic and agronomic variation in arabinoxylan and ferulic acid contents of durum wheat (Triticum durum L.) grain and its milling fractions. Journal of Cereal Science, 25, 103–110.
Levine, A., Tenhaken, R., Dixon, R., & Lamb, C. (1994). H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell, 79, 583–593.
Mahoney, N., & Molyneux, R. J. (2004). Phytochemical inhibition of aflatoxigenicity in Aspergillus flavus by constituents of walnut (Juglans regia). Journal of Agricultural and Food Chemistry, 52, 1882–1889.
Mallozzi, M. A. B., Correa, B., Haraguchi, M., & Neto, F. B. (1996). Effect of flavonoids on Aspergillus flavus growth and aflatoxin production. Revista de Microbiologia, 27, 161–165.
Manoharan, M., Dahleen, L. S., Hohn, T. M., Neate, S. M., Yu, X. H., Alexander, N. J., et al. (2006). Expression of 3-OH trichothecene acetyltransferase in barley (Hordeum vulgare L.) and effects on deoxynivalenol. Plant Science, 171, 699–706.
Matern, U., & Kneusel, R. E. (1988). Phenolic compounds in plant disease resistance. Phytoparasitica, 16, 153–170.
McCormick, S. P., Alexander, N. J., Trapp, S. E., & Hohn, T. M. (1999). Disruption of TRI101, the gene encoding trichothecene 3-O-acetyltransferase, from Fusarium sporotrichioides. Applied and Environmental Microbiology, 65, 5252–5256.
McCormick, S. P., Bhatnagar, D., Goynes, W. R., & Lee, L. S. (1988). An inhibitor of aflatoxin biosynthesis in developing cottonseed. Canadian Journal of Botany, 66, 998–1002.
McKeehen, J. D., Bush, R. H., & Fulcher, R. G. (1999). Evaluation of wheat (Triticum aestivum L.) phenolic acids during grain development and their contribution to Fusarium resistance. Journal of Agricultural and Food Chemistry, 47, 1476–1482.
Mesterházy, Á. (2002). Role of deoxynivalenol in aggressiveness of Fusarium graminearum and F. culmorum and in resistance to Fusarium head blight. European Journal of Plant Pathology, 108, 675–684.
Mesterházy, Á., Bartók, T., Mirocha, C. G., & Komoróczy, R. (1999). Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant Breeding, 118, 97–110.
Miller, J. D., & Arnison, P. G. (1986). Degradation of deoxynivalenol by suspension cultures of the Fusarium head blight resistant wheat cultivar Frontana. Canadian Journal of Plant Pathology, 8, 147–150.
Miller, J. D., & Blackwell, B. A. (1986). Biosynthesis of 3-acetyldeoxynivalenol and other metabolites by Fusarium culmorum HLX 1503 in a stirred jar fermentor. Canadian Journal of Botany, 64, 1–5.
Miller, J. D., Fielder, D. A., Dowd, P. F., Norton, R. A., & Collins, F. W. (1996). Isolation of 4-acetyl-benzoxazolin-2-one (4-ABOA) and diferuloylputrescine from an extract of Gibberella ear rot-resistant corn that blocks mycotoxin biosynthesis, and the insect toxicity of 4-ABOA and related compounds. Biochemical Systematics and Ecology, 24, 647–658.
Miller, J. D., & Young, J. C. (1985). Deoxynivalenol in an experimental Fusarium graminearum infection of wheat. Canadian Journal of Plant Pathology, 7, 132–134.
Miller, J. D., Young, J. C., & Sampson, D. R. (1985). Deoxynivalenol and Fusarium head blight resistance in spring cereals. Phytopathologische Zeitschrift, 113, 359–367.
Miller, J. D., Young, J. C., & Trenholm, H. L. (1983). Fusarium toxins in field corn. I. Time course of fungal growth and production of deoxynivalenol and other mycotoxins. Canadian Journal of Botany, 61, 3080–3087.
Mitterbauer, R., & Adam, G. (2002). Saccharomyces cerevisae and Arabidopsis thaliana: useful model systems for the identification of molecular mechanisms involved in resistance of plants to toxins. European Journal of Plant Pathology, 108, 699–703.
Moore, J., Liu, J. G., Zhou, K. Q., & Yu, L. L. (2006). Effects of genotype and environment on the antioxidant properties of hard winter wheat bran. Journal of Agricultural and Food Chemistry, 54, 5313–5322.
Mpofu, A., Sapirstein, H. D., & Beta, T. (2006). Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. Journal of Agricultural and Food Chemistry, 54, 1265–1270.
Muthukrishnan, S., Liang, G. H., Trick, H. N., & Gill, B. S. (2001). Pathogenesis-related proteins and their genes in cereals. Plant Cell, Tissue and Organ Culture, 64, 93–114.
Naczk, M., & Shahidi, F. (2004). Extraction and analysis of phenolics in food. Journal of Chromatography A, 1054, 95–111.
Nagarajan, V., & Bhat, R. V. (1972). Factor responsible for varietal differences in aflatoxin in maize. Journal of Agricultural and Food Chemistry, 20, 911–914.
Nesci, A. V., & Etcheverry, M. G. (2006). Control of Aspergillus growth and aflatoxin production using natural maize phytochemicals under different conditions of water activity. Pest Management Science, 62, 775–784.
Neucere, J. N., & Godshall, M. A. (1991). Effects of base-soluble proteins and methanol-soluble polysaccharides from corn on mycelial growth of Aspergillus flavus. Mycopathologia, 113, 103–108.
Nicholson, R. L., & Hammerschmidt, R. (1992). Phenolic-compounds and their role in disease resistance. Annual Review of Phytopathology, 30, 369–389.
Norton, R. A. (1997). Effect of carotenoids on aflatoxin B1 synthesis by Aspergillus flavus. Phytopathology, 87, 814–821.
Norton, R. A. (1999). Inhibition of aflatoxin B1 biosynthesis in Aspergillus flavus by anthocyanidins and related flavonoids. Journal of Agricultural and Food Chemistry, 47, 1230–1235.
Ohsato, S., Ochiai-Fukuda, T., Nishiuchi, T., Takahashi-Ando, N., Koizumi, S., Hamamoto, H., et al. (2007). Transgenic rice plants expressing trichothecene 3-O-acetyltransferase show resistance to the Fusarium phytotoxin deoxynivalenol. Plant Cell Reports, 26, 531–538.
Okubara, P. A., Blechl, A. E., McCormick, S. P., Alexander, N. J., Dill-Macky, R., & Hohn, T. M. (2002). Engineering deoxynivalenol metabolism in wheat through the expression of a fungal trichothecene acetyltransferase gene. Theoretical and Applied Genetics, 106, 74–83.
Passi, S., Nazzaro-Porro, M., Fanelli, C., Fabbri, A. A., & Fasella, P. (1984). Role of lipoperoxidation in aflatoxin production. Applied Microbiology and Biotechnology, 19, 186–190.
Pinson-Gadais, L., Barreau, C., Chaurand, M., Gregoire, S., Monmarson, M., & Richard-Forget, F. (2007). Distribution of toxigenic Fusarium spp. and mycotoxin production in milling fractions of durum wheat. Food Additives and Contaminants, 24, 53–62.
Ponts, N. (2005). Influence de stress oxydatifs sur la biosynthèse de mycotoxines de Fusarium spp. contaminantes de l’épi de maïs. Doctoral Thesis, Bordeaux University, France.
Ponts, N., Pinson-Gadais, L., Barreau, C., Richard-Forget, F., & Ouellet, T. (2007). Exogenous H2O2 and catalase treatments interfere with Tri genes expression in liquid cultures of Fusarium graminearum. FEBS Letters, 581, 443–447.
Ponts, N., Pinson-Gadais, L., & Richard-Forget, F. (2003). H2O2 effects on trichothecenes B (DON, ADON) production by Fusarium graminearum in liquid culture. Aspects of Applied Biology, 68, 223–228.
Ponts, N., Pinson-Gadais, L., Verdal-Bonnin, M. N., Barreau, C., & Richard-Forget, F. (2006). Accumulation of deoxynivalenol and its 15-acetylated form is significantly modulated by oxidative stress in liquid cultures of Fusarium graminearum. FEMS Microbiology Letters, 258, 102–107.
Poppenberger, B., Berthiller, F., Lucyshyn, D., Sieberer, T., Schuhmacher, R., Krska, R., et al. (2003). Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. Journal of Biological Chemistry, 278, 47905–47914.
Proctor, R. H., Hohn, T. M., & McCormick, S. P. (1995). Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Molecular Plant-Microbe Interactions, 8, 593–601.
Reid, L. M., Mather, D. E., Arnason, J. T., Hamilton, R. I., & Bolton, A. T. (1992). Changes in phenolic constituents of maize silk infected with Fusarium graminearum. Canadian Journal of Botany, 70, 1697–1702.
Repka, V. (1999). Improved histochemical test for in situ detection of hydrogen peroxide in cells undergoing oxidative burst or lignification. Biologia Plantarum, 42, 599–607.
Rocha, O., Ansari, K., & Doohan, F. M. (2005). Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Additives and Contaminants, 22, 369–378.
Savard, M. E. (1991). Deoxynivalenol fatty acid and glucoside conjugates. Journal of Agricultural and Food Chemistry, 39, 570–574.
Schatzmayr, G., Zehner, F., Taubel, M., Schatzmayr, D., Klimitsch, A., Loibner, A. P., et al. (2006). Microbiologicals for deactivating mycotoxins. Molecular Nutrition and Food Research, 50, 543–551.
Schneweis, I., Meyer, K., Engelhardt, G., & Bauer, J. (2002). Occurrence of zearalenone-4-beta-D-glucopyranoside in wheat. Journal of Agricultural and Food Chemistry, 50, 1736–1738.
Schroeder, H. W., & Christensen, J. J. (1963). Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology, 53, 831–838.
Scott, P. M., Nelson, K., Kanhere, S. R., Karpinski, K. F., Hayward, S., Neish, G. A., et al. (1984). Decline in deoxynivalenol (vomitoxin) concentrations in 1983 Ontario winter wheat before harvest. Applied and Environmental Microbiology, 48, 884–886.
Sewald, N., Vongleissenthall, J. L., Schuster, M., Muller, G., & Aplin, R. T. (1992). Structure elucidation of a plant metabolite of 4-desoxynivalenol. Tetrahedron-Asymmetry, 3, 953–960.
Siranidou, E., Kang, Z., & Buchenauer, H. (2002). Studies on symptom development, phenolic compounds and morphological defence responses in wheat cultivars differing in resistance to Fusarium head blight. Journal of Phytopathology, 150, 200–208.
Snijders, C. H. A. (2004). Resistance in wheat to Fusarium infection and trichothecene formation. Toxicology Letters, 153, 37–46.
Swanson, S. P., Helaszek, C., Buck, W. B., Rood Jr., H. D., & Haschek, W. M. (1988). The role of intestinal microflora in the metabolism of trichothecene mycotoxins. Food and Chemical Toxicology, 26, 823–829.
Swanson, S. P., Rood Jr., H. D., Behrens, J. C., & Sanders, P. E. (1987). Preparation and characterization of the deepoxy trichothecenes: deepoxy HT-2, deepoxy T-2 triol, deepoxy T-2 tetraol, deepoxy 15-monoacetoxyscirpenol and deepoxy scirpentriol. Applied and Environmental Microbiology, 53, 2821–2826.
Van Loon, L. C., & Van Strien, E. A. (1999). The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiological and Molecular Plant Pathology, 55, 85–97.
Vergopoulou, S., Galanopoulou, D., & Markaki, P. (2001). Methyl jasmonate stimulates aflatoxin B1 biosynthesis by Aspergillus parasiticus. Journal of Agricultural and Food Chemistry, 49, 3494–3498.
Vigers, A. J., Roberts, W. K., & Selitrennikoff, C. P. (1991). A new family of plant antifungal proteins. Molecular Plant-Microbe Interactions, 4, 315–323.
Wakulinski, W. (1989). Phytotoxicity of the secondary metabolites of fungi causing wheat head fusariosis (head blight). Acta Physiologiae Plantarum, 11, 301–306.
Wallace, G., & Fry, S. C. (1994). Phenolic components of the plant cell wall. International Review of Cytology, 151, 229–267.
Wang, Y. Z., & Miller, J. D. (1988). Effects of Fusarium graminearum metabolites on wheat tissue in relation to Fusarium head blight resistance. Journal of Phytopathology, 122, 118–125.
Wicklow, D. T., Norton, R. A., & McAlpin, C. E. (1998). β-Carotene inhibition of aflatoxin biosynthesis among Aspergillus flavus genotypes from Illinois corn. Mycoscience, 39, 167–172.
Wu, X., Murphy, P., Cunnick, J., & Hendrich, S. (2007). Synthesis and characterization of deoxynivalenol glucuronide: its comparative immunotoxicity with deoxynivalenol. Food and Chemical Toxicology, 45, 1846–1855.
Yao, Q., Liu, Z., & Zeng, Y. (1996). Detoxification of deoxynivalenol by scab resistant wheat and the bioactivities of the product. Acta Mycologica Sinica, 15, 59–64.
Zeringue Jr., H. J., Brown, R. L., Neucere, J. N., & Cleveland, T. E. (1996). Relationships between C6-C12 alkanal and alkenal volatile contents and resistance of maize genotypes to Aspergillus flavus and aflatoxin production. Journal of Agricultural and Food Chemistry, 44, 403–407.
Zhou, W. C., Kolb, F. L., & Riechers, D. E. (2005). Identification of proteins induced or upregulated by Fusarium head blight infection in the spikes of hexaploid wheat (Triticum aestivum). Genome, 48, 770–780.
Acknowledgements
This work is part of Anne-Laure Boutigny’s PhD project financially supported by the IRTAC (Institut de Recherches Technologiques Agroalimentaires des Céréales), the ANRT (Association Nationale de la Recherche Technique), and the ‘Ministère de l’Enseignement supérieur et de la Recherche’ as part of the National Integrated Research Project ‘RARE fusariotoxines 2003–2007’. We would like to thank Thérèse Ouellet and Shea Miller for review of an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Boutigny, AL., Richard-Forget, F. & Barreau, C. Natural mechanisms for cereal resistance to the accumulation of Fusarium trichothecenes. Eur J Plant Pathol 121, 411–423 (2008). https://doi.org/10.1007/s10658-007-9266-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-007-9266-x