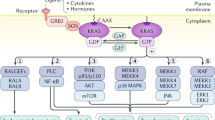
Abstract
The diagnostic utility of detecting K-ras mutations for the diagnosis of exocrine pancreatic cancer (EPC) has not been properly studied, and few reports have analysed a clinically relevant spectrum of patients. The objective was to evaluate the clinical validity of detecting K-ras mutations in the diagnosis of EPC in a large sample of clinically relevant patients. We prospectively identified 374 patients in whom one of the following diagnoses was suspected at hospital admission: EPC, chronic pancreatitis, pancreatic cysts, and cancer of the extrahepatic biliary system. Mutations in the K-ras oncogene were analysed by PCR and artificial RFLP in 212 patients. The sensitivity and specificity of the K-ras mutational status for the diagnosis of EPC were 77.7% (95% CI: 69.2–84.8) and 78.0% (68.1–86.0), respectively. The diagnostic accuracy was hardly modified by sex and age. In patients with either mutated K-ras or CEA > 5 ng/ml, the sensitivity and specificity were 81.0% (72.9–87.6) and 62.6% (72.9–87.6), respectively. In patients with mutated K-ras and CEA > 5 ng/ml the sensitivity was markedly reduced. In comparisons with a variety of non-EPC patient groups sensitivity and specificity were both always greater than 75%. In this clinically relevant sample of patients the sensitivity and specificity of K-ras mutations were not sufficiently high for independent diagnostic use. However, it seems premature to rule out the utility of K-ras analysis in conjunction with other genetic and ‘omics’ technologies.

Similar content being viewed by others
Abbreviations
- BPD:
-
Benign pancreatic disease
- CEBS:
-
Cancer of the extra-hepatic biliary system
- CI:
-
Confidence interval
- CP:
-
Chronic pancreatitis
- EPC:
-
Exocrine pancreatic cancer
- ERCP:
-
Endoscopic retrograde cholangio-pancreatography
- IQR:
-
Interquartile range
- OBD:
-
Other benign disease
- OM:
-
Other malignancy
- OR:
-
Odds ratio
References
Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–72.
Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17.
Takhar AS, Palaniappan P, Dhingsa R, Lobo DN. Recent developments in diagnosis of pancreatic cancer. BMJ. 2004;329:668–73.
Porta M, Fabregat X, Malats N, Guarner L, Carrato A, de Miguel A, et al. Exocrine pancreatic cancer: symptoms at presentation and their relation to tumour site and stage. Clin Transl Oncol. 2005;7:189–97.
Duffy MJ, Sturgeon C, Lamerz R, Haglund C, Holubec VL, Klapdor R, et al. Tumor markers in pancreatic cancer: an European group on tumor markers (EGTM) status report. Ann Oncol. 2010;21:441–7.
Freelove R, Walling A. Pancreatic cancer: diagnosis and management. Am Fam Physician. 2006;73:485–92.
Beger HG, Matsuno S, Cameron JL. Diseases of the pancreas: current surgical therapy. Berlin: Springer; 2008.
von Hoff DD, Evans DB, Hruban RH, editors. Pancreatic cancer. Boston: Jones and Bartlett; 2005.
Howard JM, Idezuki Y, Ihse I, Prinz RA, editors. Surgical diseases of the pancreas. 3rd ed. Baltimore: Williams and Wilkins; 1998.
Beger HG, Warshaw AL, Büchler MW, Carr-Locke DL, Neoptolemos JP, Russell C, Sarr MG, editors. The pancreas. Vol. 2. Oxford: Blackwell; 1998.
Abraham SC, Wilentz RE, Yeo CJ, Sohn TA, Cameron JL, Boitnott JK, et al. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: are they all ‘chronic pancreatitis’? Am J Surg Pathol. 2003;27:110–20.
Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1998;53:549–54.
Tada M, Omata M, Ohto M. Clinical application of ras gene mutation for diagnosis of pancreatic adenocarcinoma. Gastroenterology. 1991;100:233–8.
Parker LA, Lumbreras B, Hernández-Aguado I, Porta M. How useful is it clinically to analyse the K-ras mutational status for the diagnosis of exocrine pancreatic cancer? A systematic review and meta-analysis. Eur J Clin Invest. 2011 (in press).
Marchese R, Muleti A, Pasqualetti P, Bucci B, Stigliano A, Brunetti E, et al. Low correspondance between K-ras mutations in pancreatic câncer tissue and detection of K-ras mutations in circulating DNA. Pancreas. 2006;32:171–7.
Wenger FA, Zieren J, Peter FJ, Jacobi CA, Müller JM. K-ras mutations in tissue and stool samples from patients with pancreatic cancer and chronic pancreatitis. Langenbecks Arch Surg. 1999;384:181–6.
Trümper L, Menges M, Daus H, Köhler D, Reinhard JO, Sackmann M, et al. Low sensitivity of the ki-ras polymerase chain reaction for diagnosing pancreatic cancer from pancreatic juice and bile: a multicenter prospective trial. J Clin Oncol. 2002;20:4331–7.
Liu TH, Wang ZY, Cui QC. Significance of the detection of Ki-ras codon 12 mutation in the diagnosis of pancreatic carcinoma. Int J Surgical Path. 1995;3:93–100.
Van Laethem JL, Vertongen P, Deviere J, Van Rampelbergh J, Rickaert F, Cremer M, Robberecht P. Detection of c-Ki-ras gene codon 12 mutations from pancreatic duct brushings in the diagnosis of pancreatic tumours. Gut. 1995;36:781–7.
Zheng M, Liu LX, Zhu AL, Qi SY, Jiang HC, Xiao ZY. K-ras gene mutation in the diagnosis of ultrasound guided fine-needle biopsy of pancreatic masses. World J Gastroenterol. 2003;9:188–91.
Mora J, Puig P, Boadas J, Urgell E, Montserrat E, Lerma E, et al. K-ras gene mutations in the diagnosis of fine-needle aspirates of pancreatic masses: prospective study using two techniques with different detection limits. Clin Chem. 1998;44:2243–8.
Shibata D, Almoguera C, Forrester K, Dunitz J, Martin SE, Cosgrove MM, et al. Detection of c-K-ras mutations in fine needle aspirates from human pancreatic adenocarcinomas. Cancer Res. 1990;50:1279–83.
Villanueva A, Reyes G, Cuatrecasas M, Martínez A, Erill N, Lerma E, et al. Diagnostic utility of K-ras mutations in fine-needle aspirates of pancreatic masses. Gastroenterology. 1996;110:1587–94.
Pugliese V, Pujic N, Saccomanno S, Gatteschi B, Pera C, Aste H, Ferrara GB, Nicolò G. Pancreatic intraductal sampling during ERCP in patients with chronic pancreatitis and pancreatic cancer: cytologic studies and k-ras-2 codon 12 molecular analysis in 47 cases. Gastrointest Endosc. 2001;54:595–9.
Van Laethem JL, Bourgeois V, Parma J, Delhaye M, Cochaux P, Velu T, et al. Relative contribution of Ki-ras gene analysis and brush cytology during ERCP for the diagnosis of biliary and pancreatic diseases. Gastrointest Endosc. 1998;47:479–85.
Parker LA, Gómez Saez N, Lumbreras B, Porta M, Hernández-Aguado I. Methodological deficits in diagnostic research using ‘-omics’ technologies: Evaluation of the QUADOMICS tool and quality of recently published studies. PLoS One. 2010;5(7):e11419.
Fletcher RH, Fletcher SW. Clinical epidemiology. The essentials. 4th ed. Philadelphia: Lippincott, Williams and Wilkins; 2005.
Haynes RB, Sackett DL, Guyatt GH, Tugwell P. Clinical epidemiology. How to do clinical practice research. 3rd ed. Philadelphia: Lippincott, Williams and Wilkins; 2005.
Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–61.
Porta M, ed. A dictionary of epidemiology. 5th ed. New York: Oxford University Press; 2008. p. 66, 69, 191, 201, 227, 233–6, 255, 258.
Porta M, Malats N, Jariod M, Grimalt JO, Rifà J, Carrato A, et al. Serum concentrations of organochlorine compounds and K-ras mutations in exocrine pancreatic cancer. Lancet. 1999;354:2125–9.
Soler M, Malats N, Porta M, Fernandez E, Guarner L, Maguire A, et al. Medical conditions in patients with pancreatic and biliary diseases: validity and agreement between data from questionnaires and medical records. Dig Dis Sci. 1999;44:2469–77.
Porta M, Costafreda S, Malats N, Guarner L, Soler M, Gubern JM, et al. Validity of the hospital discharge diagnosis in epidemiologic studies of biliopancreatic pathology. Eur J Epidemiol. 2000;16:533–41.
Porta M, Malats N, Alguacil J, Ruiz L, Jariod M, Carrato A, et al. Coffee, pancreatic cancer, and K-ras mutations: updating the research agenda. J Epidemiol Community Health. 2000;54:656–9.
Real FX, Malats N, Lesca G, Porta M, Chopin S, Lenoir GM, et al. Family history of cancer and germline BRCA2 mutations in sporadic exocrine pancreas cancer. Gut. 2002;50:653–7.
Alguacil J, Porta M, Malats N, Kauppinen T, Kogevinas M, Benavides FG, et al. Occupational exposure to organic solvents and K-ras mutations in exocrine pancreatic cancer. Carcinogenesis. 2002;23:101–6.
Morales E, Porta M, Vioque J, López T, Mendez MA, Pumarega JA, et al. Food and nutrient intakes and K-ras mutations in exocrine pancreatic cancer. J Epidemiol Community Health. 2007;61:641–9.
Crous-Bou M, De Vivo I, Porta M, Pumarega JA, López T, Alguacil J, et al. CYP1B1 polymorphisms and K-ras mutations in patients with pancreatic ductal adenocarcinoma. Dig Dis Sci. 2008;53:1417–21.
Crous-Bou M, Porta M, López T, Jariod M, Malats N, Alguacil J, et al. Lifetime history of tobacco consumption and K-ras mutations in exocrine pancreatic cancer. Pancreas. 2007;35:135–41.
Crous-Bou M, Porta M, López T, Jariod M, Malats N, Morales E, et al. Lifetime history of alcohol consumption and K-ras mutations in pancreatic ductal adenocarcinoma. Environ Mol Mutagen. 2009;50:421–30.
Crous-Bou M, Porta M, Morales E, López T, Carrato A, Puigdomènech E, et al. Past medical conditions and K-ras mutations in pancreatic ductal adenocarcinoma: a hypothesis-generating study. Cancer Causes Control. 2009;20:591–9.
Porta M, López T, Pumarega J, Jariod M, Crous-Bou M, Marco E, et al. In pancreatic ductal adenocarcinoma blood concentrations of some organochlorine compounds and coffee intake are independently associated with KRAS mutations. Mutagenesis. 2009;24:513–21.
Gasull M, Porta M, Pumarega J, Vioque J, Bosch de Basea M, Puigdomènech E, et al. The relative influence of diet and serum concentrations of organochlorine compounds on K-ras mutations in exocrine pancreatic cancer. Chemosphere. 2010;79:686–97.
Crous-Bou M. Clinical and environmental influences on the prevalence of mutations in the K-ras oncogene in patients with pancreatic ductal adenocarcinoma. Doctoral dissertation [Dir.: Porta M], Universitat Autònoma de Barcelona, Barcelona, Spain; 2009. (http://www.imim.es/programesrecerca/epidemiologia/en_documentsgrecm.html [accessed 23 Nov 2010].
Porta M, Pumarega J, López T, Jariod M, Marco E, Grimalt JO. Influence of tumor stage, symptoms and time of blood draw on serum concentrations of organochlorine compounds in exocrine pancreatic cancer. Cancer Causes Control. 2009;20:1893–906.
Porta M, Ferrer-Armengou O, Pumarega J, López T, Crous-Bou M, Alguacil A, et al. Exocrine pancreatic cancer clinical factors were related to timing of blood extraction and influenced serum concentrations of lipids. J Clin Epidemiol. 2008;61:695–704.
Armitage P, Berry G, Matthews JNS. Statistical methods in medical research. 4th ed. Blackwell: Oxford; 2002.
Porta M, Pumarega J, Ferrer-Armengou O, López T, Alguacil J, Malats N, et al. Timing of blood extraction in epidemiologic and proteomic studies: results and proposals from the PANKRAS II Study. Eur J Epidemiol. 2007;22:577–88.
Gudjonsson B. Pancreatic cancer: survival, errors and evidence. Eur J Gastroenterol Hepatol. 2009;21:1379–82.
Acknowledgments
Supported in part by research grants from the Government of Catalonia (2009 SGR 1350, CIRIT 1999 SGR 00241 and 1998/BEAi400011); CIBER de Epidemiología y Salud Pública (CIBERESP), ‘Red temática de investigación cooperativa de centros en cáncer’ (C03/10), ‘Red temática de investigación cooperativa de centros en Epidemiología y salud pública’ (C03/09), and Fondo de Investigación Sanitaria (91/595, 92/0007, 95/0017 and 97/1138), Instituto de Salud Carlos III, Madrid, Government of Spain. The authors gratefully acknowledge scientific and technical assistance provided by Antonio Salas, Montserrat Andreu, Josep Lluís Piñol, Angels Serrat, David J. MacFarlane, Laura Ruiz, Elisabeth Carrillo, José Pumarega, Marta Crous-Bou, Leo Español, Puri Barbas and Yolanda Rovira.
Conflict of interest
The authors declare they have no competing financial interests nor other conflicts of interest. The study sponsors had no role and no involvement in the study design, nor in the collection, analysis, and interpretation of data; they also had no role and no involvement in the writing of the report, nor in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
For the PANKRAS II Study Group: Members of the Multicentre Prospective Study on the Role of the K-ras and other Genetic Alterations in the Diagnosis, Prognosis and Etiology of Pancreatic and Biliary Diseases (PANKRAS II) Study Group are mentioned in previous publications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Parker, L.A., Porta, M., Lumbreras, B. et al. Clinical validity of detecting K-ras mutations for the diagnosis of exocrine pancreatic cancer: a prospective study in a clinically-relevant spectrum of patients. Eur J Epidemiol 26, 229–236 (2011). https://doi.org/10.1007/s10654-011-9547-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-011-9547-8