Abstract
Buccal cells are an important source of DNA in epidemiological studies, but little is known about factors that influence amount and purity of DNA. We assessed these factors in a self-administered buccal cell collection procedure, obtained with three cotton swabs. In 2,451 patients DNA yield and in 1,033 patients DNA purity was assessed. Total DNA yield ranged from 0.08 to 1078.0 μg (median 54.3 μg; mean 82.2 μg ± SD 92.6). The median UV 260:280 ratio, was 1.95. Samples from men yielded significantly more DNA (median 58.7 μg) than those from women (median 44.2 μg). Diuretic drug users had significantly lower purity (median 1.92) compared to other antihypertensive drug users (1.95). One technician obtained significantly lower DNA yields. Older age was associated with lower DNA purity. In conclusion, DNA yield from buccal swabs was higher in men and DNA purity was associated with age and the use of diuretics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidemiologists are increasingly trying to supplement observational data with biological material, including DNA. Blood is the specimen of choice for obtaining genomic DNA for most large scale epidemiological studies [1, 2]. However, such studies might need alternative sources when study subjects are reluctant to provide a blood sample or when only a self-administered collection protocol is logistically or economically feasible. Buccal cell collection seems to be a good alternative for invasive blood collection. This buccal cell collection can be performed by a buccal swab or mouth wash procedure. A few studies compared these methods in terms of DNA-yield. Most of these studies found that mouthwash procedures provide more yield and higher-quality DNA than buccal swab methods [2–4]. Nevertheless, DNA collection with the use of buccal swabs has many advantages such as light weight postage, cost effective processing for long-term archiving, and easy obtain ability from widely dispersed participants, it is comfortable for the patient and tasteless. Moreover, collecting buccal cells rather than blood may be especially appropriate in a pediatric setting. Furthermore buccal swab methods provide sufficient DNA for polymerase chain reactions in which only nanogram quantities of DNA are needed [5, 6]. However, little is known about factors that influence the amount and purity of DNA, obtained from buccal cell collection protocols. Knowledge of these factors is of great importance to optimize the yield of this method. In blood, predictors of variation in DNA yield are age, daily smoking status, high-density lipoprotein cholesterol, systolic blood pressure, triglycerides, history of acute myocardial infarction (MI) and possibly diabetes mellitus [7, 8]. Determinants of DNA yield from whole blood samples may not be the same as for buccal samples. Results from studies that focused on determinants of DNA yield in buccal cells are inconsistent and considered only a few factors while as far as we know, no study has focused on drug use as a determinant of DNA yield and no study has assessed determinants of DNA purity [1, 9, 10]. Therefore, we conducted a study to assess which factors determine the amount and purity of DNA in a self-administered, non-invasive and relatively inexpensive buccal cell collection procedure.
Materials and methods
Study design
We designed a case–control study in which we will assess whether specific genetic polymorphisms modify the effect of antihypertensive drugs. Within this large pharmacogenetic study we conducted a cross-sectional study to perform determinants of DNA yield and purity [11–13]. Participants were enrolled from the population-based pharmaco-morbidity record linkage system (PHARMO). PHARMO has been linking drug dispensing histories from a representative sample of Dutch community pharmacies to the national registration of hospital discharges (LMR) as from 1985. In the PHARMO database, subjects who use antihypertensive drugs were selected. From this cohort, subjects hospitalized for MI were included as cases. Controls, without MI, were matched on age (±1 year), sex and geographical location. Patients were excluded if they were <18 years of age, if they were not currently taking at least 1 antihypertensive medication at the date of hospitalization for the first MI for cases (last prescription not more than 100 days before index date; 90 days plus 10 days to account for irregularity of refills) and the same date for their matched controls or if their DNA yield was not available.
Procedures
For all patients information about the use of drugs, that induce hyposalivation as an adverse drug reaction (anticholinergics ATC code: R03BB, antidepressants ATC code: N06A, anti-inflammatory and antirheumatic products, non-steroid ATC code: M01A, topical products for joint and muscular pain ATC code: M02A, benzodiazepine derivatives ATC code: N05BA, antipsychotics ATC code: N05A, sympathomimetics ATC code: R01BA, muscle relaxants peripherally acting agents ATC code: M03), on the date of DNA collection was obtained from the PHARMO database [14, 15]. Patients were recruited through community pharmacies, which participate in PHARMO. From these pharmacies the patients received a letter in which the purpose of the study was explained. They were asked for written informed consent to participate in this study and to return the informed consent form and a questionnaire. For all participants explicit informed consent was asked for collection, storage and genotyping of the buccal swab material. Information on ethnicity, smoking, hypertension, hypercholesterolemia, diabetes mellitus, use of alcohol, diets, history of cardiovascular diseases, family history of cardiovascular diseases, weight and height was collected through self-administered questionnaires and linked with automated general practice and laboratory registrations. Approval for this study was obtained from the Medical Ethics Committee of the University Medical Center Utrecht, The Netherlands.
DNA collection and isolation
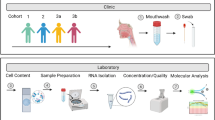
Individuals who agreed to participate in the study were asked to supply a sample of buccal cells. The collection of buccal swabs was performed by the participants themselves [14, 15]. They received one page of collection instructions, three cotton swabs, and three 15 ml tubes containing 2 ml buffer (1,880 μl STE (100 mM NaCl, 10 mM EDTA, 10 mM Tris), 100 μl 10% SDS and 20 μl of 10 mg/ml Proteinase K). All participants were instructed to rinse their mouth with tap water before collection. Subsequently, they were instructed to rub with the swab along the inside of the cheek and against their gums for 1 min in the morning, afternoon and evening. After each rub the cotton swab should be placed in one of the tubes and then sent back, in a prepaid return envelope, to the laboratory. Upon arrival, the Proteinase K concentration was increased to 0.2 mg/ml and the sample was lysed by incubation at 65°C for 2 h. The cotton swabs were placed in a syringe cover inside a 50 ml tube and centrifuged at 1,000 rpm for 60 s. The remaining buffer from the original 15 ml tube was poured into the 50 ml tube. DNA was then purified by adding 0.2 volumes of potassium acetate and putting the sample on ice for 15 min. The aqueous phase was extracted with 1 volume chloroform/isoamyl alcohol (24:1) and mixed for 30 min. After 15 min of centrifuging at 3,000 rpm the aqueous phase was transferred to a clean 50 ml tube. DNA was precipitated by adding two volumes ethanol absolute and pelleted by centrifugation (3,000 rpm for 10 min). After washing with 70% ethanol twice, the pellet was dried and resuspended in TE (200 μl). DNA samples were stored at −30°C.
The yield and purity of the DNA were determined by spectrophotometry (nanodrop ND-1000) using the ratio of UV absorbance at 260 and 280 nm. A UV absorbance ratio of 1.8 < R < 2.0 was considered to be good purified DNA. The UV absorbance ratio measurements were started up later in time. Laboratory personnel were blinded to patient characteristics.
We did not know at what time the patient was collecting the DNA and whether the patient returned the swabs immediately after the collection to the laboratory. Therefore, in order to assess the influence of time from swabbing to extraction on DNA yield and purity, buccal swab samples from the same person (n = 4) were collected and stored for 2 weeks, for 1 week, for 1 day and for a few hours in the collection medium (buffer and enzyme) before DNA was isolated. For these samples we also performed a gel electrophoresis analysis.
Statistical analysis
Median regression was used to examine the relationship between median DNA yield (and DNA purity) and determinants, 95% confidence intervals for regression coefficients were estimated by inverting a rank test as described in Koenker [16].
All analyses were performed using R 2.8.1 with library “quantreg”.
Results
In 2,451 patients (1,684 male and 767 female) DNA yield and in 1,033 patients (682 male and 351 female) DNA purity was assessed. The baseline characteristics are given in Table 1. There was no difference between the group from which only DNA yield was estimated and the group from which also DNA purity was estimated. The mean age of the participants was 64.5 years (range 28.5–92.5). Total DNA yield ranged from 0.08 to 1,078.0 μg (median 54.3 μg; mean ± SD is 82.2 μg ± 92.6). The median UV 260:280 ratio was 1.95, whereas 59.8% of the samples had a UV 260:280 ratio between 1.8 and 2.0. No association was found between DNA yield and DNA purity (data not shown). The mean time between sending buccal swabs to the patient and returning of the DNA-samples to the laboratory (=transport time) was 12 days. Samples from men yielded statistically significantly more DNA (median 58.7 μg) than those from women (median 44.2 μg; median difference 15.3 μg; Table 2). DNA purity was the same for both men and women. With increasing age there was a trend for decreasing DNA purity. Case–control status did not influence the DNA amount and DNA purity (Tables 2, 3). Laboratory technician number four obtained a lower DNA yield than the other three, which was statistically significant. The number of subjects of non-Whites origin was too few to assess racial differences in DNA yield and DNA purity. Neither the use of thiazide diuretics compared to the use of other antihypertensive drugs nor the use of other drugs that may induce hyposalivation influenced DNA yield. Nevertheless, the use of thiazide diuretics was significantly associated with decreased DNA purity compared to other antihypertensive drug use (1.92 vs. 1.95, respectively; Table 3). However, other drugs, inducing hyposalivation, did not influence DNA purity. For all analyses adjustment for the other determinants did not substantially influence the median difference.
Storage time from the swabs in the collection medium did not influence DNA yield and purity (data not shown). Furthermore, no difference in degradation was found for different storage times. In most samples only DNA with a high molecular weight was present and DNA was hardly degraded.
All DNA samples were assayed by a PCR method for genotyping of polymorphisms in the α-adducin, angiotensin converting enzyme, angiotensinogen, angiotensin II type 1 receptor, G-protein β3 and endothelial nitric oxide synthase genes [17].
Discussion
In this study, we found that DNA yield of buccal cell samples was higher in men than in women. DNA purity was associated with age and the use of thiazide diuretics.
In one study it was found that men from one study group had a higher median amount of human specific DNA compared to women who participated in another study group using a mouthwash protocol [9]. In another study which also investigated the feasibility of collecting buccal cells with a mouthwash procedure, mean DNA yield was also found to be lower in women than in men [1]. On the other hand one study did not find a significantly difference in the average amount of DNA between men and women [10]. The difference in the amount of DNA between men and women may reflect less vigorous scraping among women. However, in a study comparing 30 s rubbing the cheeks against the teeth versus no rubbing prior to a cytobrush collection did not find a difference [18].
We did not find an association between age and DNA yield. One study found a positive correlation between age and quantity of DNA in buccal cell samples and in another study variation by age in buccal cell yield was suggested [9, 10]. In blood increasing age led to a significant reduction in DNA yield [8]. This decline in DNA yield may represent the known decline in total leukocyte and lymphocyte count that occurs between birth and adulthood and is not an explanation for a decrease in buccal cell DNA yield. Dry mouth as a symptom of getting older is also not a probable explanation, considering that drugs that induce hyposalivation did not influence DNA yield.
We could not assess the influence of race on DNA yield due to a small number of black and Asian subjects. One other study concluded that mean DNA yield was lower in Japanese compared with Whites, whereas another study only suggested variation by ethnicity [1, 9].
DNA yield can vary by laboratory personnel for instance depending on the routine of the laboratory technician. The critical step in the protocol is the separation of the water phase with DNA from the chloroform/isoamyl alcohol phase. The layer between the phases may not be touched because that may influence yield and purity. A lower yield may be associated with a laboratory technician that extracts less water phase and thereby has smaller risk of touching the in between layer.
In our study no significant association with transport time was found while in one study it was stated that holding DNA mouthwash samples at room temperature and processing them 10 or 30 days after collection yielded statistically significantly less DNA [9]. The main difference between the studies consisted of the storage medium in which the DNA was transported. In our study participants used cotton swabs to collect buccal cells and were asked to place them in tubes containing buffer solution. The composition of the buffer, including proteinase K, was different from the Scope mouthwash, which was used in the other study. Storage of the swabs in tubes with buffer and enzyme for 2 weeks did not influence DNA yield compared to storage for 1 week, 1 day or no storage. The small number of samples (N = 4) used to determine the influence of the storage time is a limitation. On the other hand the median DNA yield and purity of 85 samples with a transport time longer than 30 days did not deviate from the samples with a transport time shorter than 30 days, which confirmed the findings with the four samples.
Drugs that cause hyposalivation and dry mouth as an adverse drug reaction did not influence DNA yield or DNA purity. On the other hand lower DNA purity among diuretic drug users compared to other antihypertensive drug users was found while diuretic drugs also may induce hyposalivation. It is unclear by which mechanism diuretic drugs can influence DNA purity. In our study all patients are taking antihypertensive drugs. However, DNA yield is probably not influenced by taking antihypertensive drugs, as the use of diuretic drugs, which are most likely to influence DNA yield because of their adverse drug reaction, did not modify DNA yield. For pharmacogenetic studies it is good to know that antihypertensive drugs and drugs inducing hyposalivation do not influence DNA yield. We used the PHARMO database to assess antihypertensive drug use. The PHARMO data have been validated on several occasions with regard to hospital discharge data [19, 20] and drug exposure [21, 22].
Most studies investigated the DNA purity by gel electrophoresis or PCR analysis. Only one study estimated the average DNA ratio 260:280 which was significantly higher in swab samples than in mouthwash samples. However, they did not study other determinants [2].
Two small studies mentioned the possibility of a higher DNA yield when swabbing multiple times [23, 24]. In our study the participants were asked to do the collection procedure three times which resulted in DNA yields high enough for genotyping. Collecting more than three samples will probably result in lower response rates because of unwillingness to swab repeatedly over several time points.
In our study a wide range of DNA yields was found. A possible explanation for this wide range might be the contamination with bacterial DNA. We could not differentiate between bacterial and human DNA. However, the isolated DNA was PCRable and genotyping of the samples was successful, indicating the total amount of human DNA is sufficient and also indicating the DNA samples were not too degraded. The findings of the gel electrophoresis support this non-degradation. In our study a lot of genotyping can be done with the remaining DNA. However, buccal cell samples provide substantially smaller quantities of DNA than do whole blood specimens. The whole-genome amplification (WGA) might be an attractive solution to this problem [25, 26]. The way we have isolated DNA is time-consuming. Therefore we recommend to use DNA self-collection kits which simplify DNA purification, for example oragene DNA self collection kits from DNA genotek.
In conclusion, gender, age, diuretic drug use and laboratory personnel must be taken into account when collecting buccal cell samples. To get high DNA yield and high DNA purity, it is necessary to have good qualified laboratory personnel. More over, one must consider if women need to collect an extra buccal swab.
References
Le Marchand L, et al. Feasibility of collecting buccal cell DNA by mail in a cohort study. Cancer Epidemiol Biomarkers Prev. 2001;10(6):701–3.
Cozier YC, Palmer JR, Rosenberg L. Comparison of methods for collection of DNA samples by mail in the black women’s health study. Ann Epidemiol. 2004;14(2):117–22. doi:10.1016/S1047-2797(03)00132-7.
Steinberg K, et al. DNA banking for epidemiologic studies: a review of current practices. Epidemiology. 2002;13(3):246–54. doi:10.1097/00001648-200205000-00003.
Garcia-Closas M, et al. Collection of genomic DNA from adults in epidemiological studies by buccal cytobrush and mouthwash. Cancer Epidemiol Biomarkers Prev. 2001;10(6):687–96.
Clark DW, et al. Linking pharmacovigilance with pharmacogenetics. Drug Saf. 2004;27(15):1171–84. doi:10.2165/00002018-200427150-00002.
Mulot C, et al. Collection of human genomic DNA from buccal cells for genetics studies: comparison between cytobrush, mouthwash, and treated card. J Biomed Biotechnol. 2005;2005(3):291–6. doi:10.1155/JBB.2005.291.
Alanne M, et al. DNA extraction yield is associated with several phenotypic characteristics: results from two large population surveys. J Thromb Haemost. 2004;2(11):2069–71. doi:10.1111/j.1538-7836.2004.00982.x.
Richardson AJ, et al. Blood storage at 4 degrees C-factors involved in DNA yield and quality. J Lab Clin Med. 2006;147(6):290–4. doi:10.1016/j.lab.2006.01.005.
Feigelson HS, et al. Determinants of DNA yield and quality from buccal cell samples collected with mouthwash. Cancer Epidemiol Biomarkers Prev. 2001;10(9):1005–8.
Vandenbergh DJ, Anthony K, Whitfield KE. Optimizing DNA yield from buccal swabs in the elderly: attempts to promote buccal cell growth in culture. Am J Hum Biol. 2003;15(5):637–42. doi:10.1002/ajhb.10177.
Lievers KJA, et al. Interactions between genetic polymorphisms and antihypertensive drugs in the treatment of hypertension. Eur J Hum Genet. 2004;12(1):303.
van Wieren-de Wijer DB, Maitland-van der Zee AH, de Boer A, Stricker BH, Kroon AA, de Leeuw PW, et al. Recruitment of participants through community pharmacies for a pharmacogenetic study of antihypertensive drug treatment. Pharm World Sci. 2009;31(2):158–64. doi:10.1007/s11096-008-9264-x.
van Wieren-de Wijer DB, Maitland-van der Zee AH, de Boer A, Kroon AA, de Leeuw PW, Schiffers P, et al. Interaction between the Gly460Trp alpha-adducin gene variant and diuretics on the risk of myocardial infarction. J Hypertens. 2009;27(1):61–8. doi:10.1097/HJH.0b013e328317a74d.
Tredwin CJ, Scully C, Bagan-Sebastian JV. Drug-induced disorders of teeth. J Dent Res. 2005;84(7):596–602. doi:10.1177/154405910508400703.
Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. 2003;134(1):61–9. quiz 118-9.
Koenker RW. Confidence intervals for regression quantiles. In: Mandl P, Huskova M, editors. Asymptotic statistics. New York: Springer; 1994. p. 349–59.
Knaapen AM, et al. Simultaneous genotyping of nine polymorphisms in xenobiotic-metabolizing enzymes by multiplex PCR amplification and single base extension. Clin Chem. 2004;50(9):1664–8. doi:10.1373/clinchem.2004.034058.
King IB, et al. Buccal cell DNA yield, quality, and collection costs: comparison of methods for large-scale studies. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1130–3.
De Bruin ML, van Hemel NM, Leufkens HG, Hoes AW. Hospital discharge diagnoses of ventricular arrhythmias and cardiac arrest were useful for epidemiologic research. J Clin Epidemiol. 2005;58(12):1325–9. doi:10.1016/j.jclinepi.2005.04.009.
van de Garde EM, Oosterheert JJ, Bonten M, Kaplan RC, Leufkens HG. International classification of diseases codes showed modest sensitivity for detecting community-acquired pneumonia. J Clin Epidemiol. 2007;60(8):834–8. doi:10.1016/j.jclinepi.2006.10.018.
Klungel OH, de Boer A, Paes AH, Herings RM, Seidell JC, Bakker A. Agreement between self-reported antihypertensive drug use and pharmacy records in a population-based study in The Netherlands. Pharm World Sci. 1999;21(5):217–20. doi:10.1023/A:1008741321384.
Klungel OH, de Boer A, Paes AH, Herings RM, Seidell JC, Bakker A. Influence of question structure on the recall of self-reported drug use. J Clin Epidemiol. 2000;53(3):273–7. doi:10.1016/S0895-4356(99)00167-5.
Freeman B, et al. DNA by mail: an inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behav Genet. 1997;27(3):251–7. doi:10.1023/A:1025614231190.
Meulenbelt I, et al. High-yield noninvasive human genomic DNA isolation method for genetic studies in geographically dispersed families and populations. Am J Hum Genet. 1995;57(5):1252–4.
Zheng S, et al. Whole genome amplification increases the efficiency and validity of buccal cell genotyping in pediatric populations. Cancer Epidemiol Biomarkers Prev. 2001;10(6):697–700.
Beckett SM, et al. Buccal swabs and treated cards: methodological considerations for molecular epidemiologic studies examining pediatric populations. Am J Epidemiol. 2008;167(10):1260–7. doi:10.1093/aje/kwn012.
Acknowledgments
This study was financially supported by the Netherlands Heart Foundation, grant number NHF-2001.064.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van Wieren-de Wijer, D.B.M.A., Maitland-van der Zee, A.H., de Boer, A. et al. Determinants of DNA yield and purity collected with buccal cell samples. Eur J Epidemiol 24, 677–682 (2009). https://doi.org/10.1007/s10654-009-9388-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-009-9388-x