Abstract
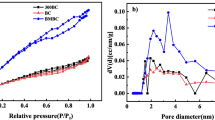
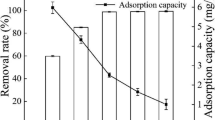
Novel biochar was prepared by ball milling using bamboo as raw material. The aim of this study was to find a good alternative way to improve the potentials of biochar for ammonium adsorption from aqueous solution. The sorption performance of ball-milled bamboo biochar (BMBB) was compared with that of bamboo biochar (BB) using batch adsorption experiments. Different adsorption kinetics models proved that the pseudo-second order was the best kinetic model for explanation of the adsorption kinetics characteristics, indicative of the energetically heterogeneous solid surface of the biochar. The Langmuir model could fit the isothermal adsorption data of BMBB well. The maximum adsorption capacity of BMBB (22.9 mg g−1) was much higher than that of BB (7.0 mg g−1). This study offers a relatively cost-effective and efficient methodology for the improvement in the adsorption capacity of biochar for ammonium nitrogen.





Similar content being viewed by others
References
Alshameri, A., Ibrahim, A., Assabri, A. M., Lei, X., Wang, H., & Yan, C. (2014). The investigation into the ammonium removal performance of Yemeni natural zeolite: Modification, ion exchange mechanism, and thermodynamics. Powder Technology,258, 20–31.
Alshameri, A., He, H., Zhu, J., Xi, Y., Zhu, R., Ma, L., et al. (2018). Adsorption of ammonium by different natural clay minerals: Characterization, kinetics and adsorption isotherms. Applied Clay Science,159, 83–93.
Carpenter, S. R., Caraco, N. F., Correll, D. L., Howarth, R. W., Sharpley, A. N., & Smith, V. H. (1998). Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecological Applications,8(3), 559–568.
Cruz, H., Luckman, P., Seviour, T., Verstraete, W., Laycock, B., & Pikaar, I. (2018). Rapid removal of ammonium from domestic wastewater using polymer hydrogels. Scientific Reports,8(1), 2912.
Erisman, J. W., Bleeker, A., Galloway, J., & Sutton, M. S. (2007). Reduced nitrogen in ecology and the environment. Environmental Pollution,150(1), 140–149.
Foo, K. Y., & Hameed, B. H. (2010). Insights into the modeling of adsorption isotherm systems. Chemical Engineering Journal,156(1), 2–10.
Gai, X., Wang, H., Liu, J., Zhai, L., Liu, S., Ren, T., et al. (2014). Effects of feedstock and pyrolysis temperature on biochar adsorption of ammonium and nitrate. PLoS ONE,9(12), e113888.
Gao, F., Xue, Y., Deng, P., Cheng, X., & Yang, K. (2015). Removal of aqueous ammonium by biochars derived from agricultural residuals at different pyrolysis temperatures. Chemical Speciation & Bioavailability,27(2), 92–97.
Hale, S. E., Alling, V., Martinsen, V., Mulder, J., Breedveld, G. D., & Cornelissen, G. (2013). The sorption and desorption of phosphate-P, ammonium-N and nitrate-N in cacao shell and corn cob biochars. Chemosphere,91(11), 1612–1619.
Hou, J., Huang, L., Yang, Z., Zhao, Y., Deng, C., Chen, Y., et al. (2016). Adsorption of ammonium on biochar prepared from giant reed. Environmental Science and Pollution Research,23(19), 19107–19115.
Huang, H., Xiao, X., Yan, B., & Yang, L. (2010). Ammonium removal from aqueous solutions by using natural Chinese (Chende) zeolite as adsorbent. Journal of Hazardous Materials,175(1–3), 247–252.
Huang, J., Kankanamge, N. R., Chow, C., Welsh, D. T., Li, T., & Teasdale, P. R. (2018). Removing ammonium from water and wastewater using cost-effective adsorbents: A review. Journal of Environmental Sciences,63, 174–197.
Jing, Q-x, Chai, L-y, Huang, X-d, Tang, C-j, Guo, H., & Wang, W. (2017). Behavior of ammonium adsorption by clay mineral halloysite. Transactions of Nonferrous Metals Society of China,27(7), 1627–1635.
Jorgensen, T. C., & Weatherley, L. R. (2003). Ammonia removal from wastewater by ion exchange in the presence of organic contaminants. Water Research,37(8), 1723–1728.
Karapınar, N. (2009). Application of natural zeolite for phosphorus and ammonium removal from aqueous solutions. Journal of Hazardous Materials,170(2), 1186–1191.
Li, R., Wang, J. J., Zhou, B., Zhang, Z., Liu, S., Lei, S., et al. (2017). Simultaneous capture removal of phosphate, ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment. Journal of Cleaner Production,147, 96–107.
Li, X., & Zhao, Q. (2001). Efficiency of biological treatment affected by high strength of ammonium–nitrogen in leachate and chemical precipitation of ammonium–nitrogen as pretreatment. Chemosphere,44(1), 37–43.
Li, X., Zhao, Q., & Hao, X. (1999). Ammonium removal from landfill leachate by chemical precipitation. Waste Management,19(6), 409–415.
Lian, G., Wang, B., Lee, X., Li, L., Liu, T., & Lyu, W. (2019). Enhanced removal of hexavalent chromium by engineered biochar composite fabricated from phosphogypsum and distillers grains. Science of the Total Environment,697, 134119.
Limousin, G., Gaudet, J.-P., Charlet, L., Szenknect, S., Barthes, V., & Krimissa, M. (2007). Sorption isotherms: A review on physical bases, modeling and measurement. Applied Geochemistry,22(2), 249–275.
Lyu, H., Gao, B., He, F., Zimmerman, A. R., Ding, C., Huang, H., et al. (2018a). Effects of ball milling on the physicochemical and sorptive properties of biochar: Experimental observations and governing mechanisms. Environmental Pollution,233(Supplement C), 54–63.
Lyu, H., Gao, B., He, F., Zimmerman, A. R., Ding, C., Tang, J., et al. (2018b). Experimental and modeling investigations of ball-milled biochar for the removal of aqueous methylene blue. Chemical Engineering Journal,335(Supplement C), 110–119.
Ma, P. C., Wang, S. Q., Kim, J.-K., & Tang, B. Z. (2009). In-situ amino functionalization of carbon nanotubes using ball milling. Journal of nanoscience and nanotechnology,9(2), 749–753.
Ma, Z., Li, Q., Yue, Q., Gao, B., Li, W., Xu, X., et al. (2011). Adsorption removal of ammonium and phosphate from water by fertilizer controlled release agent prepared from wheat straw. Chemical Engineering Journal,171(3), 1209–1217.
Manikandan, A., Subramanian, K., & Pandian, K. (2013). Effect of high energy ball milling on particle size and surface area of adsorbents for efficient loading of fertilizer. An Asian Journal of Soil Science,8(2), 249–254.
Peterson, S. C., Jackson, M. A., Kim, S., & Palmquist, D. E. (2012). Increasing biochar surface area: Optimization of ball milling parameters. Powder Technology,228, 115–120.
Rajapaksha, A. U., Chen, S. S., Tsang, D. C., Zhang, M., Vithanage, M., Mandal, S., et al. (2016). Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere,148(27), 276–291.
Richard, S., Rajadurai, J. S., & Manikandan, V. (2016). Influence of particle size and particle loading on mechanical and dielectric properties of biochar particulate-reinforced polymer nanocomposites. International Journal of Polymer Analysis and Characterization,21(6), 462–477.
Spokas, K. A., Novak, J. M., & Venterea, R. T. (2012). Biochar's role as an alternative N-fertilizer: Ammonia capture. Plant and Soil,350(1–2), 35–42.
Vikrant, K., Kim, K.-H., Ok, Y. S., Tsang, D. C. W., Tsang, Y. F., Giri, B. S., et al. (2018). Engineered/designer biochar for the removal of phosphate in water and wastewater. Science of the Total Environment,616–617, 1242–1260.
Vu, T. M., Trinh, V. T., Doan, D. P., Van, H. T., Nguyen, T. V., Vigneswaran, S., et al. (2017). Removing ammonium from water using modified corncob-biochar. Science of the Total Environment,579, 612–619.
Wang, B., Gao, B., & Fang, J. (2017). Recent advances in engineered biochar productions and applications. Critical reviews in Environmental Science and Technology,47(22), 2158–2207.
Wang, B., Gao, B., & Wan, Y. (2018a). Entrapment of ball-milled biochar in Ca-alginate beads for the removal of aqueous Cd(II). Journal of Industrial and Engineering Chemistry,61, 161–168.
Wang, B., Gao, B., & Wan, Y. (2019a). Comparative study of calcium alginate, ball-milled biochar, and their composites on aqueous methylene blue adsorption. Environmental Science and Pollution Research,26(2), 11535–11541.
Wang, B., Gao, B., Zimmerman, A. R., Zheng, Y., & Lyu, H. (2018b). Novel biochar-impregnated calcium alginate beads with improved water holding and nutrient retention properties. Journal of Environmental Management,209, 105–111.
Wang, B., Lee, X., Theng, B. K. G., Zhang, L., Cheng, H., Cheng, J., et al. (2019b). Biochar addition can reduce NOx gas emissions from a calcareous soil. Environmental Pollutants and Bioavailability,31(1), 38–48.
Wang, B., Lehmann, J., Hanley, K., Hestrin, R., & Enders, A. (2015a). Adsorption and desorption of ammonium by maple wood biochar as a function of oxidation and pH. Chemosphere,138, 120–126.
Wang, B., Lehmann, J., Hanley, K., Hestrin, R., & Enders, A. (2016). Ammonium retention by oxidized biochars produced at different pyrolysis temperatures and residence times. RSC Advances,6(48), 41907–41913.
Wang, B., Lian, G., Lee, X., Gao, B., Li, L., Liu, T., et al. (2019c). Phosphogypsum as a novel modifier for distillers grains biochar removal of phosphate from water. Chemosphere,238, 124684.
Wang, B., Wan, Y., Zheng, Y., Lee, X., Liu, T., Yu, Z., et al. (2019d). Alginate-based composites for environmental applications: A critical review. Critical Reviews in Environmental Science and Technology,49(4), 318–356.
Wang, Q., Wang, B., Lee, X., Lehmann, J., & Gao, B. (2018c). Sorption and desorption of Pb(II) to biochar as affected by oxidation and pH. Science of the Total Environment,634, 188–194.
Wang, S., & Peng, Y. (2010). Natural zeolites as effective adsorbents in water and wastewater treatment. Chemical Engineering Journal,156(1), 11–24.
Wang, Y., Liu, S., Xu, Z., Han, T., Chuan, S., & Zhu, T. (2006). Ammonia removal from leachate solution using natural Chinese clinoptilolite. Journal of Hazardous Materials,136(3), 735–740.
Wang, Z., Guo, H., Shen, F., Yang, G., Zhang, Y., Zeng, Y., et al. (2015b). Biochar produced from oak sawdust by Lanthanum (La)-involved pyrolysis for adsorption of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−). Chemosphere,119, 646–653.
Xu, X., Zheng, Y., Gao, B., & Cao, X. (2019). N-doped biochar synthesized by a facile ball-milling method for enhanced sorption of CO2 and reactive red. Chemical Engineering Journal,368, 564–572.
Yang, F., Lee, X., Theng, B. K., Wang, B., Cheng, J., & Wang, Q. (2016). Effect of biochar addition on short-term N2O and CO2 emissions during repeated drying and wetting of an anthropogenic alluvial soil. Environmental Geochemistry and Health,39(3), 634–647.
Yang, H. I., Lou, K., Rajapaksha, A. U., Ok, Y. S., Anyia, A. O., & Chang, S. X. (2018). Adsorption of ammonium in aqueous solutions by pine sawdust and wheat straw biochars. Environmental Science and Pollution Research,25(26), 25638–25647.
Yao, Y., Gao, B., Zhang, M., Inyang, M., & Zimmerman, A. R. (2012). Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere,89(11), 1467–1471.
Yin, H., & Kong, M. (2014). Simultaneous removal of ammonium and phosphate from eutrophic waters using natural calcium-rich attapulgite-based versatile adsorbent. Desalination,351, 128–137.
Zhang, Q., Wang, J., Lyu, H., Zhao, Q., Jiang, L., & Liu, L. (2019). Ball-milled biochar for galaxolide removal: Sorption performance and governing mechanisms. Science of the Total Environment,659, 1537–1545.
Zhang, T., Ding, L., Ren, H., & Xiong, X. (2009). Ammonium nitrogen removal from coking wastewater by chemical precipitation recycle technology. Water Research,43(20), 5209–5215.
Zhang, W., & Cue, B. W. (2018). Green techniques for organic synthesis and medicinal chemistry. London: Wiley.
Zheng, X., Yang, Z., Xu, X., Shi, X., Dai, M., & Guo, R. (2018). Distillers’ grains anaerobic digestion residue biochar used for ammonium sorption and its effect on ammonium leaching from an Ultisol. Environmental Science and Pollution Research,25(15), 14563–14574.
Zheng, Y., Wang, B., Wester, A. E., Chen, J., He, F., Chen, H., et al. (2019). Reclaiming phosphorus from secondary treated municipal wastewater with engineered biochar. Chemical Engineering Journal,362, 460–468.
Zhu, K., Fu, H., Zhang, J., Lv, X., Tang, J., & Xu, X. (2012). Studies on removal of NH4+-N from aqueous solution by using the activated carbons derived from rice husk. Biomass and Bioenergy,43, 18–25.
Acknowledgements
Financial support for this work was provided by the National Key Research and Development Program of China (2016YFC0502602), the High-Level Overseas Talent Innovation and Entrepreneurship Project of Guizhou Province [Grant No. (2018)08] and the Opening Fund of State Key Laboratory of Environmental Geochemistry (SKLEG2019704).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qin, Y., Zhu, X., Su, Q. et al. Enhanced removal of ammonium from water by ball-milled biochar. Environ Geochem Health 42, 1579–1587 (2020). https://doi.org/10.1007/s10653-019-00474-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-019-00474-5