Abstract
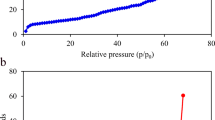
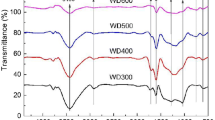
The primary objective of this study was to investigate the effect of biochar, produced from wheat residue at different temperatures, on the adsorption of diesel oil by loess soil. Kinetic and equilibrium data were processed to understand the adsorption mechanism of diesel by biochar-affected loess soil; dynamic and thermodynamic adsorption experiments were conducted to characterize this adsorption. The surface features and chemical structure of biochar, modified at varying pyrolytic temperatures, were investigated using surface scanning electron microscopy and Fourier transform infrared analysis. The kinetic data showed that the adsorption of diesel oil onto loess soil could be described by a pseudo-second-order kinetic model, with the rate-controlling step being intraparticle diffusion. However, in the presence of biochar, boundary layer control and intraparticle diffusion were both involved in the adsorption. Besides, the adsorption equilibrium data were well described by the Freundlich isothermal model. The saturated adsorption capacity weakened as temperature increased, suggesting a spontaneous exothermic process. Thermodynamic parameter analysis showed that adsorption was mainly a physical process and was enhanced by chemical adsorption. The adsorption capacity of loess soil for diesel oil was weakened with increasing pH. The biochar produced by pyrolytic wheat residue increased the adsorption behavior of petroleum pollutants in loess soil.






Similar content being viewed by others
References
Ahmad, M., Lee, S. S., Dou, X., Mohan, D., Sung, J. K., Yang, J. E., & Ok, Y. S. (2012). Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresource technology, 118, 536–544.
Ahmad, M., Lee, S. S., Rajapaksha, A. U., Vithanage, M., Zhang, M., Cho, J. S., et al. (2013). Trichloroethylene adsorption by pine needle biochars produced at various pyrolysis temperatures. Bioresource technology, 143, 615–622.
Ahmad, M., Rajapaksha, A. U., Lim, J. E., Zhang, M., Bolan, N., Mohan, D., & Ok, Y. S. (2014). Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere, 99, 19–33.
Chen, J. P., Wu, S., & Chong, K. H. (2003). Surface modification of a granular activated carbon by citric acid for enhancement of copper adsorption. Carbon, 41, 1979–1986.
Chen, Y., Xiao, B., Chang, J., Fu, Y., Lv, P., & Wang, X. (2009). Synthesis of biodiesel from waste cooking oil using immobilized lipase in fixed bed reactor. Energy Conversion and Management, 50, 668–673.
Chun, Y., Sheng, G., Chiou, C. T., & Xing, B. (2004). Compositions and sorptive properties of crop residue-derived chars. Environmental Science and Technology, 38, 4649–4655.
Delle Site, A. (2001). Factors affecting sorption of organic compounds in natural sorbent/water systems and sorption coefficients for selected pollutants, a review. Journal of Physical and Chemical Reference Data, 30, 187–439.
Dubinin, M. M., Zaverina, E. D., & Radushkevich, L. V. (1947). Sorption and structure of active carbons. I. Adsorption of organic vapors. Russian Journal of Bioorganic Chemistry, 21, 1351–1362.
Fasfous, I. I., Radwan, E. S., & Dawoud, J. N. (2010). Kinetics, equilibrium and thermodynamics of the sorption of tetrabromobisphenol A on multiwalled carbon nanotubes. Applied Surface Science, 256, 7246–7252.
Freundlich, H. (1906). Uber die adsorption in losungen (Adsorption in solution). Physical Chemistry Periodical, 57, 384–470.
Geng, C., & Lu, S. (2003). A vertical transfer regularity of oil pollutant in the soil of the northwest. Environmental Pollution and Control, 25, 61–62.
Hall, K. E., Calderon, M. J., Spokas, K. A., Cox, L., Koskinen, W. C., Novak, J., & Cantrell, K. (2014). Phenolic acid sorption to biochars from mixtures of feedstock materials. Water, Air, and Soil pollution, 225, 2031.
Helfferich, F. (1979). Ion exchange. NY: McGraw Hill.
Ho, Y. S., & McKay, G. (1998a). Sorption of dye from aqueous solution by peat. Chemical Engineering Journal, 70, 115–124.
Ho, Y. S., & McKay, G. (1998b). The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Canadian Journal of Chemical Engineering, 76, 822–827.
Ho, Y. S., & McKay, G. (1998c). Kinetic models for the sorption of dye from aqueous solution by wood. Journal of Environmental Science and Health Part B: Process Safety and Environmental Protection, 76(B2), 183–191.
Huang, C. C., Pang, J., Chen, S., Su, H., Han, J., Cao, Y., et al. (2006). Charcoal records of fire history in the Holocene loess-soil sequences over the southern Loess Plateau of China. Palaeogeography, Palaeoclimatology, Palaeoecology, 239, 28–44.
Khalladi, R., Benhabiles, O., Bentahar, F., & Moulai-Mostefa, N. (2009). Surfactant remediation of diesel fuel polluted soil. Journal of Hazardous Materials, 164, 1179–1184.
Kiran, I., Akar, T., Ozcan, A. S., Ozcan, A., & Tunali, S. (2006). Biosorption kinetics and isotherm studies of Acid Red 57 by dried Cephalosporium aphidicola cells from aqueous solutions. Biochemical Engineering Journal, 31, 197–203.
Kumari, K. G. I. D., Moldrup, P., Paradelo, M., & de Jonge, L. W. (2014). Phenanthrene sorption on biochar-amended soils: Application rate, aging, and physicochemical properties of soil. Water, Air, and Soil pollution, 225, 2015.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of American Chemical Society, 40, 1361–1403.
Liu, Y. (2009). Is the free energy change of adsorption correctly calculated? Journal of Chemical and Engineering Data, 54, 1981–1985.
Liu, W. J., Zeng, F. X., Jiang, H., & Zhang, X. S. (2011). Preparation of high adsorption capacity bio-chars from waste biomass. Bioresource technology, 102, 8247–8252.
McKay, G., Blair, H. S., & Gardener, J. R. (1982). Adsorption of dyes on chitin I, equilibrium studies. Journal of Applied Polymer Science, 27, 3043–5307.
Mohan, D., Sarswat, A., Ok, Y. S., & Pittman, C. U, Jr. (2014). Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresource technology, 160, 191–202.
Özcan, A., Özcan, A. S., Tunali, S., Akar, T., & Kiran, I. (2005). Determination of the equilibrium, kinetic and thermodynamic parameters of adsorption of copper (II) ions onto seeds of Capsicum annuum. Journal of Hazardous Materials, B124, 200–208.
Pradubmllk, T., Óhaber, J. H., Malakul, P., & Harwell, J. H. (2003). Effect of pH on adsolubilization of toluene and acetophenone into adsorbed surfactant on precipitated silica. Colloid Surface, 224, 93–98.
Rutigliano, F. A., Romano, M., Marzaioli, R., Baglivo, I., Baronti, S., Miglietta, F., & Castaldi, S. (2014). Effect of biochar addition on soil microbial community in a wheat crop. European Journal of Soil Biology, 60, 9–15.
Studzińska, S., Sprynskyy, M., & Buszewski, B. (2008). Study of sorption kinetics of some ionic liquids on different soil types. Chemosphere, 71, 2121–2128.
Sun, K., Jin, J., Keiluweit, M., Kleber, M., Wang, Z., Pan, Z., & Xing, B. (2012). Polar and aliphatic domains regulate sorption of phthalic acid esters (PAEs) to biochars. Bioresource technology, 118, 120–127.
Sun, K., Keiluweit, M., Kleber, M., Pan, Z., & Xing, B. (2011). Sorption of fluorinated herbicides to plant biomass-derived biochars as a function of molecular structure. Bioresource technology, 102, 9897–9903.
Sun, L., Wan, S., & Luo, W. (2013). Biochars prepared from anaerobic digestion trsidue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Bioresource Technology, 140, 406–413.
Troy, S. M., Lawlor, P. G., O’ Flynn, C. J., & Healy, M. J. (2014). The impact of biochar addition on nutrient leaching and soil properties from tillage soil amended with pig manure. Water, Air, and Soil pollution, 225, 1900.
Uchimiya, M., Chang, S., & Klasson, K. T. (2011). Screening biochars for heavy metal retention in soil: Role of oxygen functional groups. Journal of Hazardous Materials, 190, 432–441.
Uchimiya, M., Wartelle, L. H., Lima, I. M., & Klasson, K. T. (2010). Sorption of deisopropylatrazine on broiler litter biochars. Journal of Agricultural and Food Chemistry, 58, 12350–12356.
Weber, W. J, Jr, & Morriss, J. C. (1963). Kinetics of adsorption on carbon from solution. Journal of Sanitary Engineering Division: American Society of Civil Engineering, 89, 31–60.
Xu, R. K., Xiao, S. C., Yuan, J. H., & Zhao, A. Z. (2011). Adsorption of methyl violet from aqueous solutions by the biochars derived from crop residues. Bioresource technology, 102, 10293–10298.
Yang, Y., & Sheng, G. (2003). Enhanced pesticide sorption by soils containing particulate matter from crop residue burns. Environmental Science and Technology, 37, 3635–3639.
Yu, Y., Zhuang, Y. Y., Wang, Z. H., & Qiu, M. Q. (2004). Adsorption of water soluble days onto modified resin. Chemosphere, 54, 425–430.
Zhang, P., Sun, H., Yu, L., & Sun, T. (2013). Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: Impact of structural properties of biochars. Journal of Hazardous Materials, 244–245, 217–224.
Zhang, J. H., & Zeng, J. H. (2007). Sorption of diesel oil and its influence factor on Beijing soils. Research of Environmental Science, 20, 19–23. (in Chinese).
Zhao, X., Ouyang, W., Hao, F., Lin, C., Wang, F., Han, S., & Geng, X. (2013). Properties comparison of biochars from corn straw with different pretreatment and sorption behaviour of atrazine. Bioresource technology, 147, 338–344.
Zhao, D., Zhang, J., Duan, E., & Wang, J. (2008). Adsorption equilibrium and kinetics of dibenzothiophene from n-octane on bamboo charcoal. Applied Surface Science, 254, 3242–3247.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21067005) and the National Natural Science Foundation of China (41363008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, Y.F., Sun, H., Yves, U.J. et al. Impact of biochar produced from post-harvest residue on the adsorption behavior of diesel oil on loess soil. Environ Geochem Health 38, 243–253 (2016). https://doi.org/10.1007/s10653-015-9712-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-015-9712-1