Abstract
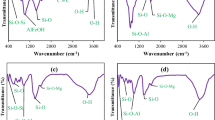
Adsorption of As(V) on various clay minerals including kaolinite (KGa-1), montmorillonite (SWy-1) and nontronites (NAU-1 and NAU-2), and subsequent bioreduction of sorbed As(V) to As(III) by bacterium Shewanella putrefaciens strain CN-32 were investigated. Nontronites showed relatively higher sorption capacity for As(V) primarily due to higher iron oxide content. Freundlich equation well described the sorption of As(V) on NAU-1, NAU-2 and SWy-1, while As(V) sorption isotherm with KGa-1 fitted well in the Langmuir model. The bacterium rapidly reduced 50 % of dissolved As(V) to As(III) in 2 h, followed by its complete reduction (>ca. 98 %) within 12 h. In contrast, sorption of As(V) to the mineral surfaces interferes with the activity of bacterium, resulting in low bioreduction of As(V) by 27 % for 5 days of incubation. S. putrefaciens also promoted the reduction of Fe(III) present in the clay mineral to Fe(II). This study indicates that the sorption and subsequent bioreduction of As(V) on clay minerals can significantly influence the mobility of As(V) in subsurface environment.





Similar content being viewed by others
References
Ahmann, D., Krumholz, L. R., Hemond, H. F., Lovley, D. R., & Morel, F. M. M. (1997). Microbial mobilization of arsenic from sediments of the Aberjona Watershed. Environmental Science and Technology, 31(10), 2923–2930.
Bentley, R., & Chasteen, T. G. (2002). Microbial methylation of metalloids: Arsenic, antimony, and bismuth. Microbiology and Molecular Biology Reviews, 66(2), 250–271.
Campbell, K. M., Malasarn, D., Saltikov, C. W., Newman, D. K., & Hering, J. G. (2006). Simultaneous microbial reduction of iron(III) and arsenic[V] in suspensions of hydrous ferric oxide. Environmental Science and Technology, 40, 5950–5955.
Cummings, D. E, Jr., Caccavo, F., Fendorf, S., & Rosenzweig, R. F. (1999). Arsenic mobilization by the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY. Environmental Science and Technology, 33, 723–729.
Dong, H. (2012). Clay–microbe interactions and implications for environmental mitigation. Elements, 8, 113–118.
Dong, H., Kukkadapu, R., Fredrickson, J. K., Zachara, J. M., Kennedy, D. W., & Kostandarithes, H. M. (2003). Microbial reduction of structural Fe(III) in illite and Goethite. Environmental Science and Technology, 37, 1268–1276.
Fendorf, S., Eich, M. J., Grossl, P., & Sparks, D. L. (1997). Arsenate-73 uptake by components of several acidic soils and its implication for phosphate retention. Environmental Science and Technology, 31, 315–320.
Gates, W. P., Slade, P. G., Manceau, A., & Lanson, B. (2002). Site occupancies by iron in nontronites. Clays and Clay Minerals, 50, 223–239.
Halajnia, A., Oustan, S., Najafi, N., Khataee, A. R., & Lakzian, A. (2012). The adsorption characteristics of nitrate on Mg–Fe and Mg–Al layered double hydroxides in a simulated soil solution. Applied Clay Science, 70, 28–36.
Herbel, M. J., & Fendorf, S. (2006). Biogeochemical processes controlling the speciation and transport of arsenic within iron coated sands. Chemical Geology, 228, 16–32.
Huang, J. H., Voegelin, A., Pombo, S. A., Lazzaro, A., Zeyer, J., & Kretzschmar, R. (2011). Influence of arsenate adsorption to ferrihydrite, goethite, andboehmite on the kinetics of arsenate reduction by Shewanella putrefaciens strain CN-32. Environmental Science and Technology, 45, 7701–7709.
Jaisi, D. P., Kukkadapu, R. K., Eberl, D. D., & Dong, H. (2005). Control of Fe(III) site occupancy on the rate and extent of microbial reduction of Fe(III) in nontronite. Geochimica et Cosmochimica Acta, 69, 5429–5440.
Jeon, B. H., Dempsey, B. A., Burgos, W. D., Barnett, M. O., & Roden, E. E. (2005). Chemical reduction of U(VI) by Fe(II) at the solid water interface using natural and synthetic Fe(III) oxides. Environmental Science and Technology, 39, 5642–5649.
Jeon, B. H., Dempsey, B. A., Royer, R. A., & Burgos, W. D. (2004a). Low-temperature oxygen trap for maintaining strict anoxic conditions. Journal of Environmental Engineering, 130(11), 1407–1410.
Jeon, B. H., Kelly, S. D., Kemner, K. M., Barnett, M. O., Burgos, W. D., Dempsey, B. A., & Roden, E. E. (2004b). Microbial reduction of U(VI) at the solid water interface. Environmental Science and Technology, 38, 5649–5655.
Jiang, S., Lee, J. H., Kim, D., Kanaly, R. A., Kim, M. G., & Hur, H. G. (2013). Differential arsenic mobilization from As-bearing ferrihydrite by iron-respiring Shewanella strains with different arsenic-reducing activities. Environmental Science and Technology, 47, 8616–8623.
Joanne, M. S., Lindsay, I. S., Aimin, W., Dean, C., De Pascal, W. D., & Joan, M. M. (2002). New arsenite-oxidizing bacteria isolated from Australian gold mining environments phylogenetic relationships. Geomicrobiology Journal, 19, 67–76.
Jones, C. A., Langner, H. W., Anderson, K., McDermott, T. R., & Inskeep, W. P. (2000). Rates of microbially mediated arsenate reduction and solubilization. Soil Science Society of America Journal, 64, 600–608.
Keeling, J. L., Raven, M. D., & Gates, W. P. (2000). Geology and characterization of two hydrothermal nontronites from weathered metamorphic rocks at the Uley graphite mine, South Australia. Clays and Clay Minerals, 48, 537–548.
Kocar, B. D., Borch, T., & Fendorf, S. (2010). Arsenic repartitioning during biogenic sulfidization and transformation of ferrihydrite. Geochimica et Cosmochimica Acta, 74, 980–994.
Kocar, B. D., Polizzotto, M. L., Benner, S. G., Ying, S. C., Ung, M., Ouch, K., et al. (2008). Integrated biogeochemical and hydrologic processes driving arsenic release from shallow sediments to groundwaters of the Mekong delta. Applied Geochemistry, 23, 3059–3071.
Lee, S. H., Jung, W., Jeon, B. H., Choi, J. Y., & Kim, S. (2011). Abiotic subsurface behaviors of As(V) with Fe(II). Environmental Geochemistry and Health, 33, 13–22.
Lee, J. H., Roh, Y., Kim, K. W., & Hur, H. G. (2007). Organic acid dependent iron mineral formation by a newly isolated iron-reducing bacterium, Shewanella sp. HN-41. Geomicrobiology Journal, 24, 31–41.
Lin, Z., & Puls, R. W. (2000). Adsorption, desorption and oxidation of arsenic affected by clay minerals and aging process. Environmental Geology, 39, 753–759.
Lovley, D. (2013). Dissimilatory Fe(III)- and Mn(IV)-reducing prokaryotes. In E. D. Rosenberg, E. F. Stackebrandt, S. Lory & F. Thompson (Eds.), The prokaryotes–prokaryotic physiology and biochemistry (pp. 287–308). Heidelberg: Springer.
Manning, B. A., & Goldberg, S. (1996). Modeling arsenate competitive adsorption on kaolinite, montmorillonite and illite. Clays and Clay Minerals, 44, 609–623.
Manning, B. A., & Goldberg, S. (1997). Adsorption and stability of Arsenic(III) at the clay mineral-water interface. Environmental Science and Technology, 31, 2005–2011.
Meng, X.G., & Wang, W. (1998). Speciation of arsenic by disposable cartridges. In Book of posters of the third international conference on arsenic exposure and health effects. Society of Environmental Geochemistry and Health, University of Colorado at Denver, Denver, CO.
Mohapatra, D., Mishra, D., Chaudhury, G. R., & Das, R. P. (2007). Arsenic adsorption mechanism on clay minerals and its dependence on temperature. Korean Journal of Chemical Engineering, 24, 426–430.
Nicholas, D. R., Ramamoorthy, S., Palace, V., Spring, S., Moore, J. N., & Rosenzweig, R. F. (2003). Biogeochemical transformations of arsenic in circumneutral freshwater sediments. Biodegradation, 14, 123–137.
Ona-Nguema, G., Morin, G., Wang, Y., Menguy, N., Juillot, F., Olivi, L., et al. (2009). Arsenite sequestration at the surface of nano-Fe(OH)2, ferrous-carbonate hydroxide, and green-rust after bioreduction of arsenic-sorbed lepidocrocite by Shewanella putrefaciens. Geochimica et Cosmochimica Acta, 73, 1359–1381.
Polizzotto, M. L., Kocar, B. D., Benner, S. G., Sampson, M., & Fendorf, S. (2008). Near-surface wetland sediments as a source of arsenic release to ground water in Asia. Nature, 454, 505–508.
Royer, R. A., Dempsey, B., Jeon, B. H., & Burgos, W. (2004). Inhibition of biological reductive dissolution of hematite by ferrous iron. Environmental Science and Technology, 38, 187–193.
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17, 517–568.
Stolz, J. F., & Oremland, R. S. (1999). Bacterial respiration of arsenic and selenium. FEMS Microbiology Reviews, 23, 615–627.
Stookey, L. I. (1970). Ferrozine—A new spectrophotometric regent for iron. Analytical Chemistry, 42, 779–781.
Zachara, J. M., Fredrickson, J. K., Li, S. W., Kennedy, D. W., Smith, S. C., & Gassman, P. L. (1998). Bacterial reduction of crystalline Fe(III) oxides in single phase suspension and subsurface materials. American Mineralogist, 83, 1426–1443.
Zeng, L. (2004). Arsenic adsorption from aqueous solutions on an Fe(III)–Si binary oxide adsorbent. Water Quality Research Journal of Canada, 39, 267–275.
Zhang, X., Jia, Y., & Wang, S. (2012). Bacterial reduction and release of adsorbed arsenate on Fe(III)-, Al- and coprecipitated Fe(III)/Al-hydroxides. Journal of Environmental Sciences, 24, 440–448.
Zhang, H., & Selim, H. M. (2005). Kinetics of arsenate adsorption–desorption in soils. Environmental Science and Technology, 39, 6101–6108.
Zobrist, J. (2000). Mobilization of arsenite by dissimilatory reduction of adsorbed arsenate. Environmental Science and Technology, 34, 4747–4753.
Zobrist, J., Dowdle, P. R., Davis, J. A., & Oremland, R. S. (2000). Mobilization of arsenite by dissimilatory reduction of adsorbed arsenate. Environmental Science and Technology, 34, 4747–4753.
Acknowledgments
The authors are thankful for the financial support of Ferdowsi University of Mashhad, Iran, and Mine Reclamation Corporation (MIRECO), S. Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghorbanzadeh, N., Lakzian, A., Halajnia, A. et al. Influence of clay minerals on sorption and bioreduction of arsenic under anoxic conditions. Environ Geochem Health 37, 997–1005 (2015). https://doi.org/10.1007/s10653-015-9708-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-015-9708-x