Abstract
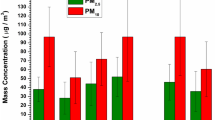
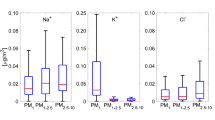
The characterization of aerosol acidity has received increased attention in recent years due to its influence on atmospheric visibility, climate change and human health. Distribution of water soluble inorganic (WSI) ions in 12 different size fractions of aerosols was investigated under two different atmospheric conditions (smoke-haze and non-haze periods) in 2012 using the Micro-Orifice Uniform Deposit Impactor (MOUDI) and nano-MOUDI for the first time in Singapore. To estimate the in situ acidity ([H+]Ins) and in situ aerosol pH (pHIS), the Aerosol Inorganic Model version-IV under deliquescent mode of airborne particles was used at prevailing ambient temperature and relative humidity. The study revealed an increase in the levels of airborne particulate matter (PM) mass and concentrations of WSI ions for all size fractions during the smoke-haze period, which was caused by the trans-boundary transport of biomass burning-impacted air masses from Indonesia. A bimodal distribution was observed for concentrations of SO4 2−, NO3 −, Cl−, K+ and Na+, whereas concentrations of NH4 +, Ca2+ and Mg2+ showed a single mode distribution. The concentration of WSI ions in PM1.8 during the smoke-haze period increased by 3.8 (for SO4 2−) to 10.5 (for K+) times more than those observed during the non-haze period. The pHIS were observed to be lower during the smoke-haze period than that during the non-haze period for all size fractions of PM, indicating that atmospheric aerosols were more acidic due to the influence of biomass burning emissions. The particles in the accumulation mode were more acidic than those in the coarse mode.






Similar content being viewed by others
References
Andreae, M. O., Andreae, T. W., Annegarn, H., Beer, J., Cachier, H., Le Canut, P., et al. (1998). Airborne studies of aerosol emissions from savanna fires in southern Africa: 2. Aerosol chemical composition. Journal of Geophysical Research: Atmospheres, 103(D24), 32119–32128.
Balasubramanian, R., Qian, W. B., Decesari, S., Facchini, M. C., & Fuzzi, S. (2003). Comprehensive characterization of PM2.5 aerosols in Singapore. Journal of Geophysical Research: Atmospheres, 108(D16), 4523. doi:10.1029/2002JD002517.
Behera, S. N., & Balasubramanian, R. (2014). Influence of biomass burning on temporal and diurnal variations of acidic gases, particulate nitrate and sulfate in a tropical urban atmosphere. Advances in Meteorology. doi:10.1155/2014/828491.
Behera, S. N., Betha, R., Liu, P., & Balasubramanian, R. (2013). A study of diurnal variations of PM2.5 acidity and related chemical species using a new thermodynamic equilibrium model. Science of the Total Environment, 452, 286–295.
Behera, S. N., & Sharma, M. (2010). Investigating the potential role of ammonia in ion chemistry of fine particulate matter formation for an urban environment. Science of the Total Environment, 408(17), 3569–3575.
Behera, S. N., & Sharma, M. (2012). Transformation of atmospheric ammonia and acid gases into components of PM2. 5: An environmental chamber study. Environmental Science and Pollution Research, 19(4), 1187–1197.
Betha, R., Behera, S. N., & Balasubramanian, R. (2014). 2013 Southeast Asian smoke haze: Fractionation of particulate-bound elements and associated health risk. Environmental Science and Technology, 48(8), 4327–4335.
Betha, R., Pradani, M., Lestari, P., Joshi, U. M., Reid, J. S., & Balasubramanian, R. (2013). Chemical speciation of trace metals emitted from Indonesian peat fires for health risk assessment. Atmospheric Research, 122, 571–578.
Cao, G., & Jang, M. (2009). An SOA model for toluene oxidation in the presence of inorganic aerosols. Environmental Science and Technology, 44(2), 727–733.
Chang, L. P., Tsai, J. H., Chang, K. L., & Lin, J. J. (2008). Water-soluble inorganic ions in airborne particulates from the nano to coarse mode: A case study of aerosol episodes in southern region of Taiwan. Environmental Geochemistry and Health, 30(3), 291–303.
Cheng, S. H., Yang, L. X., Zhou, X. H., Xue, L. K., Gao, X. M., Zhou, Y., & Wang, W. X. (2011). Size-fractionated water-soluble ions, situ pH and water content in aerosol on hazy days and the influences on visibility impairment in Jinan, China. Atmospheric Environment, 45(27), 4631–4640.
Clegg, S. L., Brimblecombe, P., & Wexler, A. S. (1998a). Thermodynamic model of the system H+–NH4 +–Na+–SO4 2−–NO3 −–Cl−–H2O at 298.15 K. The Journal of Physical Chemistry A, 102(12), 2155–2171.
Clegg, S. L., Brimblecombe, P., & Wexler, A. S. (1998b). Thermodynamic model of the system H+–NH4 +–SO4 2−–NO3–H2O at tropospheric temperatures. The Journal of Physical Chemistry A, 102(12), 2137–2154.
Draxler, R. R., & Rolph, G.D. (2013). HYSPLIT (HYbrid Single-Particle Lagrangian Integrated Trajectory) model access via NOAA ARL READY. Website http://www.arl.noaa.gov/HYSPLIT.php. College Park, MD: NOAA Air Resources Laboratory.
Du, H., Kong, L., Cheng, T., Chen, J., Du, J., Li, L., et al. (2011). Insights into summertime haze pollution events over Shanghai based on online water-soluble ionic composition of aerosols. Atmospheric Environment, 45(29), 5131–5137.
Engling, G., He, J., Betha, R., & Balasubramanian, R. (2014). Assessing the regional impact of Indonesian biomass burning emissions based on organic molecular tracers and chemical mass balance modeling. Atmospheric Chemistry and Physics, 14, 8043–8054.
Friese, E., & Ebel, A. (2010). Temperature dependent thermodynamic model of the system H+–NH4 +–Na+–SO4 2−–NO3 −–Cl−–H2O. The Journal of Physical Chemistry A, 114(43), 11595–11631.
Hatch, C. D., & Grassian, V. H. (2008). 10th anniversary review: Applications of analytical techniques in laboratory studies of the chemical and climatic impacts of mineral dust aerosol in the Earth’s atmosphere. Journal of Environmental Monitoring, 10(8), 919–934.
He, K., Zhao, Q., Ma, Y., Duan, F., Yang, F., Shi, Z., et al. (2012). Spatial and seasonal variability of PM 2.5 acidity at two Chinese megacities: Insights into the formation of secondary inorganic aerosols. Atmospheric Chemistry and Physics, 12(3), 1377–1395.
Holma, B. (1985). Influence of buffer capacity and pH-dependent rheological properties of respiratory mucus on health effects due to acidic pollution. Science of the Total Environment, 41(2), 101–123.
Jang, M., Czoschke, N. M., Lee, S., & Kamens, R. M. (2002). Heterogeneous atmospheric aerosol production by acid-catalyzed particle-phase reactions. Science, 298(5594), 814–817.
Jickells, T. D., An, Z. S., Andersen, K. K., Baker, A. R., Bergametti, G., Brooks, N., et al. (2005). Global iron connections between desert dust, ocean biogeochemistry, and climate. Science, 308(5718), 67–71.
Karthikeyan, S., & Balasubramanian, R. (2006). Determination of water-soluble inorganic and organic species in atmospheric fine particulate matter. Microchemical Journal, 82(1), 49–55.
Katsouyanni, K., Touloumi, G., Samoli, E., Gryparis, A., Le Tertre, A., Monopolis, Y., et al. (2001). Confounding and effect modification in the short-term effects of ambient particles on total mortality: Results from 29 European cities within the APHEA2 project. Epidemiology, 12(5), 521–531.
Kennish, M. J. (1994). Practical handbook of marine science. Boca Raton, FL: C.R.C. Press.
Kerminen, V. M., Hillamo, R., Teinilä, K., Pakkanen, T., Allegrini, I., & Sparapani, R. (2001). Ion balances of size-resolved tropospheric aerosol samples: Implications for the acidity and atmospheric processing of aerosols. Atmospheric Environment, 35(31), 5255–5265.
Kerminen, V. M., & Wexler, A. S. (1995). Growth laws for atmospheric aerosol particles: An examination of the bimodality of the accumulation mode. Atmospheric Environment, 29(22), 3263–3275.
Kittelson, D. B. (1998). Engines and nanoparticles: A review. Journal of Aerosol Science, 29(5), 575–588.
Lee, H. S., Kang, C. M., Kang, B. W., & Kim, H. K. (1999). Seasonal variations of acidic air pollutants in Seoul, South Korea. Atmospheric Environment, 33(19), 3143–3152.
Leiva, G. M. A., Santibañez, D. A., Ibarra, E. S., Matus, C. P., & Seguel, R. (2013). A five-year study of particulate matter (PM2.5) and cerebrovascular diseases. Environmental Pollution, 181, 1–6.
Lin, C. C., Chen, S. J., Huang, K. L., Lee, W. J., Lin, W. Y., Liao, C. J., & Chiu, C. H. (2007). Water-soluble ions in nano/ultrafine/fine/coarse particles collected near a busy road and at a rural site. Environmental Pollution, 145(2), 562–570.
Lin, Y. C., & Cheng, M. T. (2007). Evaluation of formation rates of NO2 to gaseous and particulate nitrate in the urban atmosphere. Atmospheric Environment, 41(9), 1903–1910.
Ma, J., Tang, J., Li, S. M., & Jacobson, M. Z. (2003). Size distributions of ionic aerosols measured at Waliguan observatory: Implication for nitrate gas‐to‐particle transfer processes in the free troposphere. Journal of Geophysical Research: Atmospheres, 108(D17). doi:10.1029/2002JD003356.
Manktelow, P. T., Carslaw, K. S., Mann, G. W., & Spracklen, D. V. (2010). The impact of dust on sulfate aerosol, CN and CCN during an East Asian dust storm. Atmospheric Chemistry and Physics, 10(2), 365–382.
Meng, Z., & Seinfeld, J. H. (1994). On the source of the submicrometer droplet mode of urban and regional aerosols. Aerosol Science and Technology, 20(3), 253–265.
Meng, Z., Seinfeld, J. H., Saxena, P., & Kim, Y. P. (1995). Atmospheric gas-aerosol equilibrium: IV. Thermodynamics of carbonates. Aerosol Science and Technology, 23(2), 131–154.
Menon, S., Hansen, J., Nazarenko, L., & Luo, Y. (2002). Climate effects of black carbon aerosols in China and India. Science, 297(5590), 2250–2253.
Nel, A. (2005). Air pollution-related illness: Effects of particles. Science, 308(5723), 804–806.
Nenes, A., Krom, M. D., Mihalopoulos, N., Van Cappellen, P., Shi, Z., Bougiatioti, A., et al. (2011). Atmospheric acidification of mineral aerosols: A source of bioavailable phosphorus for the oceans. Atmospheric Chemistry and Physics, 11(13), 6265–6272.
Nenes, A., Pandis, S. N., & Pilinis, C. (1998). ISORROPIA: A new thermodynamic equilibrium model for multiphase multicomponent inorganic aerosols. Aquatic Geochemistry, 4(1), 123–152.
Pathak, R. K., Louie, P. K. K., & Chan, C. K. (2004). Characteristics of aerosol acidity in Hong Kong. Atmospheric Environment, 38(19), 2965–2974.
Pathak, R. K., Yao, X., Lau, A. K., & Chan, C. K. (2003). Acidity and concentrations of ionic species of PM2.5 in Hong Kong. Atmospheric Environment, 37(8), 1113–1124.
Pavagadhi, S., Betha, R., Venkatesan, S., Balasubramanian, R., & Hande, M. P. (2013). Physicochemical and toxicological characteristics of urban aerosols during a recent Indonesian biomass burning episode. Environmental Science and Pollution Research, 20(4), 2569–2578.
Pierson, W. R., & Brachaczek, W. W. (1988). Coarse-and fine-particle atmospheric nitrate and HNO3(g) in Claremont, California, during the 1985 nitrogen species methods comparison study. Atmospheric Environment, 22(8), 1665–1668.
Pope, C. A., & Dockery, D. W. (2006). Health effects of fine particulate air pollution: Lines that connect. Journal of the Air and Waste Management Association, 56(6), 709–742.
Poppe, D., Wallasch, M., & Zimmermann, J. (1993). The dependence of the concentration of OH on its precursors under moderately polluted conditions: A model study. Journal of Atmospheric Chemistry, 16(1), 61–78.
Raizenne, M., Neas, L. M., Damokosh, A. I., Dockery, D. W., Spengler, J. D., Koutrakis, P., et al. (1996). Health effects of acid aerosols on North American children: Pulmonary function. Environmental Health Perspectives, 104(5), 506.
Reid, J. S., Hyer, E. J., Johnson, R. S., Holben, B. N., Yokelson, R. J., Zhang, J., et al. (2013). Observing and understanding the Southeast Asian aerosol system by remote sensing: An initial review and analysis for the Seven Southeast Asian Studies (7SEAS) program. Atmospheric Research, 122, 403–468.
Rengarajan, R., Sudheer, A. K., & Sarin, M. M. (2011). Aerosol acidity and secondary organic aerosol formation during wintertime over urban environment in western India. Atmospheric Environment, 45(11), 1940–1945.
Ryu, S. Y., Kim, J. E., Zhuanshi, H., Kim, Y. J., & Kang, G. U. (2004). Chemical composition of post-harvest biomass burning aerosols in Gwangju, Korea. Journal of the Air and Waste Management Association, 54(9), 1124–1137.
Saxena, P., Mueller, P. K., Kim, Y. P., Seinfeld, J. H., & Koutrakis, P. (1993). Coupling thermodynamic theory with measurements to characterize acidity of atmospheric particles. Aerosol Science and Technology, 19(3), 279–293.
See, S. W., Balasubramanian, R., Rianawati, E., Karthikeyan, S., & Streets, D. G. (2007). Characterization and source apportionment of particulate matter 2.5 μm in Sumatra, Indonesia, during a recent peat fire episode. Environmental Science and Technology, 41(3488–3494), 2007.
See, S. W., Balasubramanian, R., & Wang, W. (2006). A study of the physical, chemical, and optical properties of ambient aerosol particles in Southeast Asia during hazy and nonhazy days. Journal of Geophysical Research: Atmospheres, 111(D10). doi:10.1029/2005JD006180.
Song, C. H., & Carmichael, G. R. (1999). The aging process of naturally emitted aerosol (sea-salt and mineral aerosol) during long range transport. Atmospheric Environment, 33(14), 2203–2218.
Sun, Z., Mu, Y., Liu, Y., & Shao, L. (2013). A comparison study on airborne particles during haze days and non-haze days in Beijing. Science of the Total Environment, 456, 1–8.
Sun, Y., Zhuang, G., Tang, A., Wang, Y., & An, Z. (2006). Chemical characteristics of PM2.5 and PM10 in haze-fog episodes in Beijing. Environmental Science and Technology, 40(10), 3148–3155.
Surratt, J. D., Lewandowski, M., Offenberg, J. H., Jaoui, M., Kleindienst, T. E., Edney, E. O., & Seinfeld, J. H. (2007). Effect of acidity on secondary organic aerosol formation from isoprene. Environmental Science and Technology, 41(15), 5363–5369.
Tan, J. H., Duan, J. C., Chen, D. H., Wang, X. H., Guo, S. J., Bi, X. H., et al. (2009). Chemical characteristics of haze during summer and winter in Guangzhou. Atmospheric Research, 94(2), 238–245.
Tsai, J. H., Chang, L. P., & Chiang, H. L. (2013). Size mass distribution of water-soluble ionic species and gas conversion to sulfate and nitrate in particulate matter in Southern Taiwan. Environmental Science and Pollution Research, 20(7), 4587–4602.
Tsai, J. H., Lin, J. H., Yao, Y. C., & Chiang, H. L. (2012). Size distribution and water soluble ions of ambient particulate matter on episode and non-episode days in Southern Taiwan. Aerosol and Air Quality Research, 12, 263–274.
Tsai, H. H., Yuan, C. S., Hung, C. H., & Lin, C. (2011). Physicochemical properties of PM2. 5 and PM2.5–10 at Inland and offshore sites over Southeastern Coastal Region of Taiwan Strait. Aerosol and Air Quality Research, 11, 664–678.
Wall, S. M., John, W., & Ondo, J. L. (1988). Measurement of aerosol size distributions for nitrate and major ionic species. Atmospheric Environment, 22(8), 1649–1656.
Wang, Y., Zhuang, G., Sun, Y., & An, Z. (2006). The variation of characteristics and formation mechanisms of aerosols in dust, haze, and clear days in Beijing. Atmospheric Environment, 40(34), 6579–6591.
Weijers, E. P., Schaap, M., Nguyen, L., Matthijsen, J., Denier Van Der Gon, H. A. C., Ten Brink, H. M., & Hoogerbrugge, R. (2011). Anthropogenic and natural constituents in particulate matter in the Netherlands. Atmospheric Chemistry and Physics, 11(5), 2281–2294.
Xue, J., Lau, A. K., & Yu, J. Z. (2011). A study of acidity on PM2.5 in Hong Kong using online ionic chemical composition measurements. Atmospheric Environment, 45(39), 7081–7088.
Yao, X., Lau, A. P., Fang, M., Chan, C. K., & Hu, M. (2003). Size distributions and formation of ionic species in atmospheric particulate pollutants in Beijing, China: 1—inorganic ions. Atmospheric Environment, 37(21), 2991–3000.
Yao, X., Ling, T. Y., Fang, M., & Chan, C. K. (2007). Size dependence of in situ pH in submicron atmospheric particles in Hong Kong. Atmospheric Environment, 41(2), 382–393.
Yu, C. Y., Dong, C., Wang, X. F., Yang, L. X., & Wang, W. X. (2011). Size distributions of water-soluble inorganic ions of atmospheric aerosol particles in autumn in Jinan. Journal of Environmental Science (China), 31(4), 561–5677.
Zhang, Q., Jimenez, J. L., Worsnop, D. R., & Canagaratna, M. (2007). A case study of urban particle acidity and its influence on secondary organic aerosol. Environmental Science and Technology, 41(9), 3213–3219.
Zhou, Y., Xue, L., Wang, T., Gao, X., Wang, Z., Wang, X., et al. (2012). Characterization of aerosol acidity at a high mountain site in central eastern China. Atmospheric Environment, 51, 11–20.
Zhuang, H., Chan, C. K., Fang, M., & Wexler, A. S. (1999). Size distributions of particulate sulfate, nitrate, and ammonium at a coastal site in Hong Kong. Atmospheric Environment, 33(6), 843–853.
Ziemba, L. D., Fischer, E., Griffin, R. J., & Talbot, R. W. (2007). Aerosol acidity in rural New England: Temporal trends and source region analysis. Journal of Geophysical Research: Atmospheres, 112(D10S22). doi:10.1029/2006JD007605.
Acknowledgments
This research programme is funded by the National Research Foundation (NRF), Prime Minister’s Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) programme. The authors are grateful to NRF for the financial support from Grant No. R-706-002-101-281.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Behera, S.N., Cheng, J. & Balasubramanian, R. In situ acidity and pH of size-fractionated aerosols during a recent smoke-haze episode in Southeast Asia. Environ Geochem Health 37, 843–859 (2015). https://doi.org/10.1007/s10653-014-9660-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-014-9660-1