Abstract
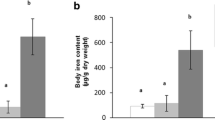
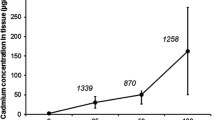
While arsenic is toxic to all multicellular organisms, some organisms become tolerant by an unknown mechanism. We have recently uncovered an inducible tolerance mechanism in insects, which is based on a sequestration of toxins and pathogens by lipid particles. To examine whether arsenic interacts with lipid particles from mammals we compared binding of arsenic to lipid particles from insect and pig plasma after separation of lipid particles by low-density gradient centrifugation. Arsenic was found in both organisms in an area of the gradient, which corresponds to lipid-rich lipid particles. Since iron is known to affect arsenic toxicity in some organisms, we asked whether iron may be present in lipid particles. When low density cell (LDC) gradient fractions were analysed for the presence of iron we detected a peak in very low-density fractions similar to those that carried arsenic. This could indicate that arsenic interacts with lipid particles that contain iron and, if arsenic is removed from the plasma by lipid particles, that would also reduce iron-containing lipid particles at the time of arsenic emergence in the plasma. To test this assumption we measured the iron content in plasma at various time periods after the toxin ingestion. This time course revealed that iron is depleted in plasma fractions when arsenic shows a peak. Our data suggest that arsenic interacts with invertebrate and vertebrate lipid particles that are associated with proteins that may lead to detoxification by cell-free or cellular sequestration mechanisms.




Similar content being viewed by others
References
Canavoso, L. E., Jouni, Z. E., Karnas, K. J., Pennington, J. E., & Wells, M. A. (2001). Fat metabolism in insects. Annual Review of Nutrition, 21, 23–46.
Conner, S. D., & Schmid, S. L. (2003). Differential requirements for AP-2 in clathrin-mediated endocytosis. Journal of Cell Biology, 162, 773–780.
Fujimoto, L. M., Roth, R., Heuser, J. E., & Schmid, S. L. (2000). Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis. Traffic, 1, 161–171.
Georgieva, T., Dunkov, B. C., Dimov, S., Ralchev, K., & Law, J. H. (2002). Drosophila melanogaster ferritin: cDNA encoding a light chain homologue, temporal and tissue specific expression of both subunit types. Insect Biochemistry and Molecular Biology, 32, 295–302.
Karlsson, C., Korayem, A. M., Scherfer, C., Loseva, O., Dushay, M. S., & Theopold, U. (2004). Proteomic analysis of the Drosophila larval hemolymph clot. Journal of Biological Chemistry, 279, 52033–52041.
Langdon, C. J., Piearce, T. G., Meharg, A. A., & Semple, K. T. (2003). Interactions between earthworms and arsenic in the soil environment: a review. Environmental Pollution, 124, 361–373.
Ma, G. (2005). The molecular biology of tolerance to Bacillus thuringiensis endotoxin in Helicoverpa armigera: a novel mechanism and its genetic transmission. Adelaide: University of Adelaide.
Ma, G., Roberts, H., Sarjan, M., Featherstone, N., Lahnstein, J., Akhurst, R., et al. (2005). Is the mature endotoxin Cry1Ac from Bacillus thuringiensis inactivated by a coagulation reaction in the gut lumen of resistant Helicoverpa armigera larvae? Insect Biochemistry and Molecular Biology, 35, 729–739.
Nappi, A. J., & Vass, E. (2000). Iron, metalloenzymes and cytotoxic reactions. Cellular and Molecular Biology, 46, 637–647.
Nichol, H., & Locke, M. (1999). Secreted ferritin subunits are of two kinds in insects—molecular cloning of cDNAs encoding two major subunits of secreted ferritin from Calpodes ethlius. Insect Biochemistry and Molecular Biology, 29, 999–1013.
Ponka, P., & Lok, C. N. (1999). The transferrin receptor: role in health and disease. International Journal of Biochemistry and Cell Biology, 31, 1111–1137.
Rahman, M. M., Roberts, H. L. S., Sarjan, M., Asgari, S., & Schmidt, O. (2004). Induction and transmission of Bacillus thuringiensis tolerance in the flour moth Ephestia kuehniella. Proceedings of the National Academy of Sciences (USA), 101, 2696–2699.
Rahman, M. M., Ma, G., Roberts, H. S. L., & Schmidt, O. (2006). Cell-free immune reactions in insects. Journal of Insect Physiology, 52, 754–762.
Rahman, M. M., Roberts, H. L. S., & Schmidt, O. (2007). Tolerance to Bacillus thuringiensis endotoxin in immune-suppressed larvae of the flour moth Ephestia kuehniella. Journal of Invertebrate Pathology, 96, 125–132.
Ramalingam, T. S., West, A. P., Lebron, J. A., Nangiana, J. S., Hogan, T. H., Enns, C. A., et al. (2000). Binding to the transferrin receptor is required for endocytosis of HFE and regulation of iron homeostasis. Nature Cell Biology, 2, 953–957.
Schoen, A., Beck, B., Sharma, R., & Dube, E. (2004). Arsenic toxicity at low doses: epidemiological and mode of action considerations. Toxicology and Applied Pharmacology Arsenic in Biology and Medicine, 198, 253–267.
Wang, L., Xu, Z. R., Jia, X. Y., Jiang, J. F., & Han, X. Y. (2006). Effects of arsenic (As-III) on lipid peroxidation, glutathione content and antioxidant enzymes in growing pigs. Asian-Australasian Journal of Animal Sciences, 19, 727–733.
Warren, G. P., Alloway, B. J., Lepp, N. W., Singh, B., Bochereau, F. J. M., & Penny, C. (2003). Field trials to assess the uptake of arsenic by vegetables from contaminated soils and soil remediation with iron oxides. The Science of the Total Environment, 311, 19–33.
Yoshida, T., Yamauchi, H., & Fan Sun, G. (2004). Chronic health effects in people exposed to arsenic via the drinking water: dose-response relationships in review. Toxicology and Applied Pharmacology Arsenic in Biology and Medicine, 198, 243–252.
Yuan, X. M. (1999). Apoptotic macrophage-derived foam cells of human atheromas are rich in iron and ferritin, suggesting iron-catalysed reactions to be involved in apoptosis. Free Radical Research, 30, 221–231.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahman, M.M., Rahman, F., Sansom, L. et al. Arsenic interactions with lipid particles containing iron. Environ Geochem Health 31 (Suppl 1), 201–206 (2009). https://doi.org/10.1007/s10653-008-9236-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-008-9236-z