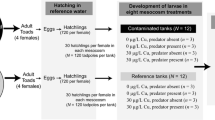
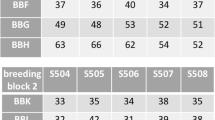
Abstract
Human-induced environmental stress may lead to rapid evolutionary processes, and can affect the ability of natural populations to respond to other environmental change or stress. We used quantitative genetics tools, pesticide exposure and a gradient of biotic stress to investigate these questions in the freshwater snail Lymnaea stagnalis. The study focused on the genetic component of variance for life-history traits within populations, and the ability of different lines to respond differently to stress. The effect of parental exposure to a xenobiotic stress on the reaction norm of the progeny to another stress was also estimated (parental non-genetic effect). First, under laboratory conditions, inter-family variance suggested significant heritability for most traits. Second, under outdoor exposure to various pesticides, variation among families was significant for individual growth. Clutch size and hatching rate of the clutches laid in the laboratory after exposure showed similar results, and moreover, family interacted significantly with pesticides. Third, under a gradient of biotic stress (food and competition), inter-family variation was again significant for growth, and a significant interaction with biotic stress was observed for juvenile growth and ultimate size. Family heterogeneity and family × environment interactions indicate the possibility of differential evolutionary responses among lines, through different reaction norms. Stressful conditions did not affect the estimated heritability, and for pesticides, no transgenerational effect was detected on progeny growth in response to the biotic stress. Focused on short-term evolutionary responses, the present study illustrates a possible way of incorporating evolutionary approaches into ecotoxicological risk assessment procedures, for example, by accounting for inter-family variation.


Similar content being viewed by others
References
Arts GHP, Buijse–Bogdan LL, Belgers JDM, Van Rhenen-Kersten CH, Van Wijngaarden RPA, Roessink I, Maund SJ, Van Den Brink PJ, Brock T (2006) Ecological impact in ditch mesocosms of simulated spray drift from a crop-protection programme for potatoes. Integr Environ Assess Manag 2:105–125
Auber A, Roucaute M, Caquet Th (2010) Effects of realistic exposure to pesticide mixtures on aquatic macroinvertebrate communities and leaf litter breakdown: a mesocosm approach. SETAC Europe 25th Annual Meeting, Seville, 23–27 May. Abstract #RA06-5
Barata C, Baird D, Mitchell SE, Soares AMVM (2002a) Among- and within-population variability in tolerance to cadmium stress in natural populations of Daphnia magna: implications for ecological risk assessment. Environ Toxicol Chem 21:1058–1064
Barata C, Baird DJ, Soares AMVM (2002b) Demographic responses of a tropical cladoceran to cadmium: effects of food supply and density. Ecol Appl 12:552–564
Beketov MA, Liess M (2005) Acute contamination with esfenvalerate and food limitation: chronic effects on the mayfly, Cloeon dipterum. Environ Toxicol Chem 24:1281–1286
Bickham JW, Sandhu S, Hebert PDN, Chikhi L, Athwal R (2000) Effects of chemicals on genetic diversity in natural populations: implications for biomonitoring and ecotoxicology. Mutat Res 463:3351
Bijlsma R, Loeschcke V (1997) Environmental stress adaptation and evolution. Birkhäuser Verlag, Basel
Bonduriansky R, Day T (2009) Nongenetic inheritance and its evolutionary implications. Annu Rev Evol Ecol Syst 40:103–125
Brown KM (1979) Effects of experimental manipulations on the life history pattern of Lymnaea stagnalis appressa Say (Pulmonata: Lymnaeidae). Hydrobiologia 65:165–176
Brown CD, van Beinum W (2009) Pesticide transport via sub-surface drains in Europe. Environ Pollut 157:3314–3324
Bubliy OA, Loeschcke V (2002) Effect of low stressful temperature on genetic variation of five quantitative traits in Drosophila melanogaster. Heredity 89:70–75
Bubliy OA, Loeschcke V, Imasheva AG (2001) Genetic variation of morphological traits in Drosophila melanogaster under poor nutrition: isofemale lines and offspring-parent regression. Heredity 86:363–369
Caquet Th, Hanson ML, Roucaute M, Graham DW, Lagadic L (2007) Influence of isolation on the recovery of pond mesocosms from the application of an insecticide. II. Benthic macroinvertebrate responses. Environ Toxicol Chem 26:1280–1290
Charlesworth B (1987) The heritability of fitness. In: Bradbury J, Anderson MB (eds) Sexual selection: testing the alternatives. John Wiley & Sons, London, pp 21–40
Coutellec MA, Lagadic L (2006) Effects of self-fertilization, environmental stress and exposure to xenobiotics on fitness-related traits of the freshwater snail Lymnaea stagnalis. Ecotoxicology 15:199–213
Coutellec MA, Caquet T, Lagadic L (2008a) Lymnaea stagnalis: the effects of experimental demographic reduction on population dynamics. In: Akçakaya HR, Stark JD, Bridges TS (eds) Demographic toxicity: methods in ecological risk assessment. Oxford University Press, Oxford, pp 152–167
Coutellec MA, Delous G, Cravedi JP, Lagadic L (2008b) Effects of the mixture of diquat and a nonylphenol polyethoxylate adjuvant on fecundity and progeny early performances of the pond snail Lymnaea stagnalis in laboratory bioassays and microcosms. Chemosphere 73:326–336
Depledge MH (1994) Genotypic toxicity: implications for individuals and populations. Environ Health Perspect 102(Suppl 12):101–104
Ducrot V, Péry ARR, Lagadic L (2010a) Modelling effects of diquat under realistic exposure patterns in genetically differentiated populations of the gastropod Lymnaea stagnalis. Phil Trans R Soc Lond S B 365:3485–3494
Ducrot V, Teixeira-Alves M, Lopes C, Delignette-Müller ML, Charles S, Lagadic L (2010b) Development of partial life-cycle experiments to assess the effects of endocrine disruptors on the freshwater gastropod Lymnaea stagnalis: a case-study with vinclozolin. Ecotoxicology 19:1312–1321
Forbes V (1999) Genetics and ecotoxicology. Taylor and Francis, Washington
Gardner MP, Fowler K, Barton NH, Partridge L (2005) Genetic variation for total fitness in Drosophila melanogaster: complex yet replicable patterns. Genetics 169:1553–1571
Hanson ML, Graham DW, Babin E, Azam D, Coutellec MA, Knapp CW, Lagadic L, Th Caquet (2007) Influence of isolation on the recovery of pond mesocosms from the application of an insecticide. I. Study design and planktonic community responses. Environ Toxicol Chem 26:1265–1279
Henry PY, Jarne P (2007) Marking hard-shelled gastropods: tag loss, impact on life history traits, and perspectives in biology. Invertebr Biol 126:138–153
Hoffmann AA, Hercus MJ (2000) Environmental stress as an evolutionary force. Bioscience 50:217–226
Hoffmann AA, Parsons PA (1997) Extreme environmental change and evolution. Cambridge University Press, Cambridge (UK)
Houle D (1992) Comparing evolvability and variability of quantitative traits. Genetics 130:195–204
Houle D, Morikawa B, Lynch M (1996) Comparing mutational variabilities. Genetics 143:1467–1483
Jarne P, David P, Pointier JP, Koene JM (2010) Pasommatophoran gastropods. In: Cordoba-Aguilar A, Leonard JL (eds) The evolution of primary sexual characters in animals. Oxford University Press, pp 173–196
Keller LF, Waller DM (2002) Inbreeding effects in wild populations. Trends Ecol Evol 17:230–241
Kirkpatrick M, Lande R (1989) The evolution of maternal characters. Evolution 43:485–503
Klerks PL (2002) Adaptation, ecological impacts, and risk assessment: insights from research at Foundry Cove, Bayou Trepagnier, and Pass Fourchon. Hum Ecol Risk Assess 8:971–982
Kruuk LEB (2004) Estimating genetic parameters in natural populations using the animal model. Phil Trans R Soc Lond B 359:873–890
Liess M (2002) Population response to toxicants is altered by intraspecific interaction. Environ Toxicol Chem 21:138–142
Long TAF, Miller PM, Stewart AD, Rice WR (2009) Estimating the heritability of female lifetime fecundity in a locally adapted Drosophila melanogaster population. J Evol Biol 22:637–643
Lynch M, Walsh B (1998) Genetics and analysis of quantitative traits. Sinauer Association Inc., Sunderland
McCullagh P, Nelder JA (1989) Generalized linear models. Chapman & Hall, London
Merilä J, Sheldon BC (1999) Genetic architecture of fitness and nonfitness traits: empirical patterns and development of ideas. Heredity 83:103–109
Morgan AJ, Kille P, Sturzenbaum SR (2007) Microevolution and ecotoxicology of metals in invertebrates. Environ Sci Technol 41:1085–1096
Mousseau TA, Uller T, Wapstra E, Badyaev AV (2009) Evolution of maternal effects: past and present. Phil Trans R Soc B 364:1035–1038
Pakkasmaa S, Merilä J, O’Hara RB (2003) Genetic and maternal effect influences on viability of common frog tadpoles under different environmental conditions. Heredity 91:117–124
Pettay JE, Kruuk LEB, Jokela J, Lummaa V (2005) Heritability and genetic constraints of life-history trait evolution in preindustrial humans. Proc Natl Acad Sci USA 102:2838–2843
Pinheiro JC, Bates DM (2002) Mixed-effects models in S and S-PLUS. Springer Verlag, New York
Price T, Schluter D (1991) On the low heritability of life-history traits. Evolution 45:853–861
Reznick DN, Ghalambor CK (2001) The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112–113:183–198
Richards CM (2000) Inbreeding depression and genetic rescue in a plant metapopulation. Am Nat 155:383–394
Ritland K (2000) Marker-inferred relatedness as a tool for detecting heritability in nature. Mol Ecol 9:1195–1204
Sgro CM, Hoffmann AA (1998) Effects of temperature extremes on genetic variance for life history traits in Drosophila melanogaster as determined from parent–offspring comparisons. J Evol Biol 11:1–20
Shugart LR, Theodorakis C (1996) Genetic ecotoxicology: the genotypic diversity approach. Comp Biochem Physiol Part C 113:273–276
Ter Maat A, Zonneveld C, de Visser JAGM, Jansen RF, Montagne-Wajer K, Koene J (2007) Food intake, growth and reproduction as affected by day length and food availability in the pond snail Lymnaea stagnalis. Am Malac Bull 23:113–120
van Straalen NM, Timmermans MJTN (2002) Genetic variation in toxicant -stressed populations: an evaluation of the “genetic erosion hypothesis”. Hum Ecol Risk Assess 8:983–1002
Whitlock MC, Ingvarsson PK, Hatfield T (2000) Local drift-load and the heterosis of interconnected populations. Heredity 84:452–457
Willi Y, van Buskirk J, Hoffmann AA (2006) Limits to the adaptative potential of small populations. Annu Rev Ecol Evol Syst 37:433–458
Acknowledgements
The present study was financially supported by the INSU EC2CO Cytrix research programme (project ‘Gaelic’). It also benefited from the experiments implemented within the ‘Emeritat’ project granted by the Ministry of Ecology, Energy, Sustainable Development and Sea through its ‘Pesticides’ research programme. The authors thank Igor Dubus, Nicolas Surdyk and Benoît Real for their involvement in the definition of treatment scenarios and for the implementation of numerical models. We thank Laurent Lagadic, coordinator of the ‘Emeritat’ research project and Virginie Ducrot, for their involvement in the experiments and for helpful discussions. We also thank Amélie d’Anfray for her contribution to the progeny experiment, the INRA group of Ecotoxicology and Quality of Aquatic Environments for collecting marked snails, and Didier Azam, Alphonse Quemeneur and the INRA Unité Expérimentale d’Ecologie Aquatique et Ecotoxicologie (Rennes, France) technical staff for support in lineages rearing, mesocosm settling and survey, and for the implementation of pesticide treatments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Coutellec, MA., Collinet, M. & Caquet, T. Parental exposure to pesticides and progeny reaction norm to a biotic stress gradient in the freshwater snail Lymnaea stagnalis . Ecotoxicology 20, 524–534 (2011). https://doi.org/10.1007/s10646-011-0611-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0611-7