Abstract
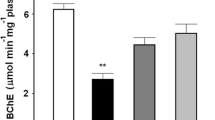
Activity of B-esterases (BChE: butyrylcholinesterase and CbE: carboxylesterase using two model substrates: α-naphthyl acetate and 4-nitrophenyl valerate) in a native frog, Leptodactylus chaquensis from rice fields (RF1: methamidophos and RF2: cypermethrin and endosulfan sprayed by aircraft) and non-contaminated area (pristine forest) was measured. The ability of pyridine-2-aldoxime methochloride (2-PAM) to reactivate BChE levels was also explored. In addition, changes in blood cell morphology and parasite infection were determined. Mean values of plasma BChE activities were lower in samples from the two rice fields than in those from the reference site. CbE (4-nitrophenyl valerate) levels varied in the three sites studied, being highest in RF1. Frog plasma from RF1 showed positive reactivation of BChE activity after incubation with 2-PAM. Blood parameters of frogs from RF2 revealed morphological alterations (anisochromasia and immature erythrocytes frequency). Moreover, a major infection of protozoan Trypanosoma sp. in individuals from the two rice fields was detected. We suggest that integrated use of several biomarkers (BChE and CBEs, chemical reactivation of plasma with 2-PAM, and blood cell parameters) may be a promising procedure for use in biomonitoring programmes to diagnose pesticide exposure of wild populations of this frog and other native anuran species in Argentina.






Similar content being viewed by others
References
Allender MC, Fry MM (2008) Amphibian hematology. Vet Clin N Am 11:463–480. doi:10.1016/j.cvex.2008.03.006
Alvisio A (1998) Arroz. Modelos zonales de producción en el movimiento CREA: Región Litoral Norte. Cuad Act Téc 61:141–145
Aranguren JD (1998) Evolución del cultivo del arroz en el movimiento CREA. Cuad Act Téc 61:6–8
Arshad N, Yunus S, Adan GE (2001) Endosulfan induced changes in hematological and some immunological parameters in mice. Punjab Univ J Zool 16:147–154
Attademo AM, Peltzer PM, Lajmanovich RC, Cabagna M, Fiorenza G (2007) Plasma B-esterases and glutathione S-transferase activities in the toad Chaunus schneideri (Amphibia, Anura) inhabiting rice agroecosystems of Argentina. Ecotoxicology 16:533–539. doi:10.1007/s10646-007-0154-0
Bagenal TB, Tesch FW (1978) Methods for assessment of a fish production in fresh 376 waters. In: Bagenal TB (ed) Age and growth, vol 377. Blackwell Scientific Publications, Oxford, pp 101–136
Bain D, Buttemer WA, Astheimer L, Fildes K, Hooper MJ (2004) Effects of sublethal fenitrothion ingestion on cholinesterase inhibition, standard metabolism, thermal preference, and prey capture ability in the Australian central bearded dragon (Pogona vitticeps: Agamidae). Environ Toxicol Chem 23:109–116. doi:10.1897/02-555
Bambaradeniya CNB, Edirisinghe JP, De Silva DN, Gunatilleke CVS, Ranawana KB, Wijekoon S (2004) Biodiversity associated with an irrigated rice agroecosystem in Sri Lanka. Biodivers Conserv 13:1715–1753. doi:0.1023/B:BIOC.0000029331.92656.de
Barni S, Boncompagni E, Grosso A, Bertone V, Freitas I, Fasola M, Fenoglio C (2007) Evaluation of Rana snk esculenta blood cell response to chemical stressors in the environment during the larval and adult phases. Aquat Toxicol 81:45–54. doi:10.1016/j.aquatox.2006.10.012
Begenesic F (1998) Arróz. Panorama Agrícola. Informe de la Secretaría de Agricultura, Ganadería, Pesca y Alimentación 2:1–47
Bunyan PJ, Jennings DM (1968) Organophosphorus poisoning; some properties of avian esterase. J Agric Food Chem 16:326–331
Burkart R, Barbaro NO, Sánchez RO, Gómez DA (1999) Eco-regiones de la Argentina. PRODIA, Buenos Aires
Busk M, Jensen FB, Wang T (2000) Effects of feeding on metabolism, gas transport and acid base balance in the bullfrog Rana Catesbiana. Am J Physiol Regul Integr Comp Physiol 278:85–95
Cabagna M, Lajmanovich RC, Stringhini G, Peltzer PM (2005) Hematological studies in the common toad (Bufo arenarum) in agrosystems of Argentina. Appl Herpetol 2:373–380. doi:10.1163/157075405774483085
Carr RL, Chambers JE (1991) Acute effects of the organophosphate paraoxon on schedule-controlled behaviour and esterase activity in rats: dose–response relationships. Pharmacol Biochem Behav 40:929–936. doi:10.1016/0091-3057(91)90108-E
CASAFE (2005) Cámara de sanidad agropecuaria y fertilizantes de la República Argentina. Guía de Productos Fitosanitarios para la República Argentina, Buenos Aires
Chambers JE, Levi PE (1992) Organophosphates: chemistry, fate and effects. Academic Press, San Diego
Chuiko GM, Podgornaya VA, Zhelnin YY (2003) Acetylcholinesterase and butyrylcholinesterase activities in brain and plasma of freshwater teleosts: cross-species and cross-family. Comp Biochem Physiol 135:55–61. doi:10.1016/S1096-4959(03)00048-4
Cordi B, Fossi C, Depledge MH (1997) Temporal biomarker responses in wild passerine birds exposed to pesticide spray drift. Environ Toxicol Chem 16:2118–2124. doi:10.1897/1551-5028(1997)016<2118:TBRIWP>2.3.CO;2
Dacie JV, Lewis SM (1984) Practical hematology. Churchill Livingstone, New York
Davidson C, Shaffer HB, Jennings MR (2002) Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate-change hypotheses for California amphibian declines. Conserv Biol 16:1588–1601. doi:10.1046/j.1523-1739.2002.01030.x
Davis AK, Maney DL, Maerz JC (2008) The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–772. doi:10.1111/j.1365-2435.2008.01467.x
DeJong RJ, Muzzall PM (2000) Hematozoa of waterfowl from the Kellogg Biological Station area in southwestern Michigan. J Wildl Dis 36:767–773
Dettbarn WD, Yang ZP, Milatovic D (1999) Different role of carboxylesterases in toxicity and tolerance to paraoxon and DFP. Chem Biol Interact 120:445–454. doi:10.1016/S0009-2797(99)00057-5
Duré MI, Kehr AI, Schaefer EF, Marangoni F (2008) Diversity of amphibians in rice fields from north-eastern Argentina. Interciencia 33:523–527
Ellman GL, Courtney KD, Andreas V Jr, Featherstone RM (1961) A new and rapid calorimetric determination of cholinesterase activity. Biochem Pharmacol 7:88–95. doi:10.1016/0006-2952(61)90145-9
Gomori G (1953) Human esterases. J Lab Clin Med 142:445–453
Grue CE, Gilbert PL, Seeley ME (1997) Neurophysiological and behavioral changes in nontarget wildlife exposed to organophosphate and carbamate pesticides: thermoregulation, food consumption, and reproduction. Am Zool 37:369–388
Guilherme S, Válega M, Pereira ME, Santos MA, Pacheco M (2008) Erythrocytic nuclear abnormalities in wild and caged fish (Liza aurata) along an environmental mercury contamination gradient. Ecotoxicol Environ Saf 70:411–421. doi:10.1016/j.ecoenv.2007.08.016
Hii YS, Lee MY, Chuah TS (2007) Acute toxicity of organochlorine insecticide endosulfan and its effect on behaviour and some hematological parameters of Asian swamp eel (Monopterus albus, Zuiew). Pestic Biochem Physiol 89:46–53. doi:10.1016/j.pestbp.2007.02.009
Iko WM, Archuleta AS, Knopf F (2003) Plasma cholinesterase levels of mountain plovers (Charadrius montanus) wintering in central California, USA. Environ Toxicol Chem 22:119–125. doi:10.1897/1551-5028(2003)022<0119:PCLOMP>2.0.CO;2
Jergentz S, Mugni H, Bonetto C, Schulz YR (2005) Assessment of insecticide contamination in runoff and stream water of small agricultural streams in the main soybean area of Argentina. Chemosphere 61:817–826. doi:10.1016/j.chemosphere.2005.04.036
Johnson PT, Chase JM (2004) Parasites in the food web: linking amphibian malformations and aquatic eutrophication. Ecol Lett 7:521–526. doi:10.1111/j.1461-0248.2004.00610.x
Kaplan HM, Glaczenski SS (1965) Hematological effects of organophosphate insecticides in the frog (Rana pipiens). Life Sci 4:1213–1219. doi:10.1016/0024-3205(65)90335-8
Khan MZ (2005) Effects of agro pesticides cypermethrin and malathion on cholinesterase activity in liver and kidney of Calotes versicolor Daudin (Agamidae: Reptilia). Turk J Zool 29:77–81
Khan MZ, Maria Z, Fatima F (2003) Effect of Lambda cyhalothrin (pyrethroid) and Monocrotophos (organophosphate) on cholinesterase activity in liver, kidney and brain of Rana cyanophlystis. Korean J Biol Sci 7:165–168
Kornienko IA, Maslov SP, Shilov IA (1965) Some general principles of adaptation in biological systems. Zh Obshch Biol 1:121–126
Laguerre C, Sánchez-Hernández JC, Kohler HR, Triebskorn R, Capowiez Y, Rault M, Mazzia C (2009) B-type esterases in the snail Xeropicta derbentina: an enzymological analysis to evaluate their use as biomarkers of pesticide exposure. Environ Pollut 157:199–207. doi:10.1016/j.envpol.2008.07.003
Lajmanovich RC, Sánchez-Hernández JC, Stringhini G, Peltzer PM (2004) Levels of serum cholinesterase activity in the Rococo toad (Bufo paracnemis) in agrosystems of Argentina. Bull Environ Contam Toxicol 72:586–591. doi:10.1007/s00128-004-0284-5
Lajmanovich RC, Sánchez-Hernández JC, Peltzer PM, Attademo AM, Fiorenza GS, Cabagna MC, Bassó A (2008) Levels of plasma B-esterases and glutathione-S-transferase activities in three South American toad species. Toxicol Environ Chem 90:1145–1161. doi:10.1080/02772240801923107
Mann RM, Hyne RV, Choung CB, Wilson SP (2009) Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ Pollut 157:2903–2927. doi:10.1016/j.envpol.2009.05.015
Marcogliese DJ, King KC, Salo HM, Fournier M, Brousseau P, Spear P, Champoux L, McLaughlin JD, Boily M (2009) Combined effects of agricultural activity and parasites on biomarkers in the bullfrog, Rana catasbeiana. Aquat Toxicol 91:126–134. doi:10.1016/j.aquatox.2008.10.001
Marques SM, Antunes SC, Pissarra H, Pereira ML, Gonçalves F, Pereira R (2009) Histopathological changes and erythrocytic nuclear abnormalities in Iberian green frogs (Rana perezi, Seoane) from a uranium mine pond. Aquat Toxicol 91:187–195. doi:10.1016/j.aquatox.2008.04.010
Maul JD, Farris JL (2005) Monitoring exposure of northern cardinals, Cardinalis cardinalis, to cholinesterase-inhibiting pesticides: enzyme activity, reactivations, and indicators of environmental stress. Environ Toxicol Chem 24:1721–1730. doi:10.1007/s00128-004-0480-3
Maxwell DM, Brecht KM (2001) Carboxylesterase: specific and spontaneous reactivation of an endogenous scavenger for organophosphorus compounds. J Appl Toxicol 21:103–107. doi:10.1002/jat.833
McCarthy J, Shugart L (1990) Biological markers of environmental contamination. In: McCarthyand J, Shugart L (eds) Biomarkers of environmental contamination. Lewis Publishers, Boca Raton, pp 3–14
Mor F, Ozmen O (2010) Endosulfan-induced neurotoxicity and serum acetylcholinesterase inhibition in rabbits: the protective effect of Vit C. Pestic Biochem Physiol 96:108–112. doi:10.1016/j.pestbp.2009.10.004
Olmos S (2006) Prácticas para el manejo de arroz Cátedra de Cultivos II. Facultad de Ciencias Agrarias, UNNE, Corrientes, Argentina
Parsons KC, Matz AC, Hooper MJ, Pokras MA (2000) Monitoring wading bird exposure to agricultural chemicals using serum cholinesterase activity. Environ Toxicol Chem 19:1317–1323. doi:10.1897/1551-5028(2000)019<1317:MWBETA>2.3.CO;2
Peltzer PM, Lajmanovich RC, Sánchez-Hernández JC, Cabagna MC, Attademo AM, Bassó A (2008) Assessment of agricultural pond eutrophication on survival and health status of the Scinax nasicus tadpoles. Environ Ecotoxicol Saf 70:185–197. doi:10.1016/j.ecoenv.2007.06.005
Pistl J, Kovalkovicova N, Kacmar P, Kosava I, Mikula I, Sutiakova I (2001) Effect of endosulfan on peripheral sheep leukocytes in vitro. Vet Hum Toxicol 43:78–82
Pough F (1980) The advantages of ectothermy for tetrapods. Am Nat 115:92–112
Ruiz A (1998) Caracterización del área arrocera de la Región CREA Litoral Norte. CREA. Cuad Act Téc 61:10–13
Sánchez JC, Fossi MC, Focardi S (1997) Serum B esterases as an nondestructive biomarker for monitoring the exposure of reptiles to organophosphorus insecticides. Ecotoxicol Environ Saf 38:45–52. doi:10.1006/eesa.1997.1560
Sánchez-Hernández JC (2003) Evaluating reptile exposure to cholinesterase-inhibiting agrochemicals by serum butyrylcholinesterase activity. Environ Toxicol Chem 22:296–301
Sánchez-Hernández JC (2006) Ecotoxicological perspectives of B-esterases in the assessment of pesticide contamination. In: Plattenberg RH (ed) Environmental pollution: new research. Nova Science Publishers, New York
Sánchez-Hernández JC, Moreno-Sánchez B (2002) Lizard cholinesterases as biomarkers of pesticide exposure: enzymological characterization. Environ Toxicol Chem 21:2319–2325. doi:10.1897/1551-5028(2002)021<2319:LCABOP>2.0.CO;2
Sánchez-Hernández JC, Carbonell R, Henríquez Pérez A, Montealegre M, Gómez L (2004) Inhibition of plasma butyrylcholinesterase activity in the lizard Gallotia galloti palmae by pesticides: a field study. Environ Pollut 132:479–488. doi:10.1016/j.envpol.2004.05.008
Silva C, Boia C, Valente J, Borrego C (2005) Pesticides in Esteros del Iberá (AR): evaluation of impacts and proposal of guidelines for water quality protection. Ecol Model 186:85–97. doi:10.1016/j.ecolmodel.2005.01.018
Sogorb MA, Vilanova E (2002) Enzymes involved in the detoxification of organophosphorus, carbamatos and pyrethroid insecticides through hydrolysis. Toxicol Lett 128:215–228. doi:10.1016/S0378-4274(01)00543-4
Soler-Rodríguez F, Miguez-Santiyan MP, Reja-Sánchez A, Roncero-Cordero V, Garcia-Cambero JP (1998) Recovery of brain acetylcholinesterase and plasma cholinesterase activities in quail (Coturnix coturnix) after chlorpyriphos administration and effect of pralidoxime treatment. Environ Toxicol Chem 17:1835–1839. doi:10.1897/1551-5028(1998)017<1835:ROBAAP>2.3.CO;2
Sparling DW, Fellers GM, McConnell L (2001) Pesticides and amphibian population declines in California USA. Environ Toxicol Chem 20:1591–1595. doi:10.1897/1551-5028(2001)020<1591:PAAPDI>2.0.CO;2
Stansley W, Roscoe DE (1996) The uptake and effects of lead in small mammals and frogs at a trap and skeet range. Arch Environ Contam Toxicol 30:220–226. doi:10.1007/BF00215801
Thompson HM, Walker CH (1994) Blood esterases as indicators of exposure to organophosphorus and carbamate insecticides. In: Fossi MC, Leonzio C, Chelsea MI (eds) Non-destructive biomarkers in vertebrates. Lewis Publishers, Boca Raton, pp 37–62
Tyler MJ (1999) Frogs and toads as experimental animals. ANZCCAR News 12:1–4
Udroiu I (2006) The micronucleus test in piscine erythrocytes. Aquat Toxicol 79:201–204. doi:10.1016/j.aquatox.2006.06.013
Varela ME, Sellarés ME (1938) Variations annuelles du sang du crepaud Bufo arenarum Hensel. C R Soc Biol 129:1248–1249
Vernadakis A, Routledge CO (1973) Effects of ether and phenobarbital anaesthesia on the activities of brain acetylcholinesterase and butyrylcholinesterase in young adult rats. J Neurochem 20:1503–1504. doi:10.1111/j.1471-4159.1973.tb00267.x
Walker CH (1998) The use of biomarkers to measure the interactive effects of chemicals. Ecotoxicol Environ Saf 40:65–70. doi:10.1006/eesa.1998.1643
Wheelock CE, Miller JL, Miller MG, Shan G, Gee SJ, Hammock BD (2004) Development of toxicity identification evaluation (TIE) procedures for pyrethroid detection using esterase activity. Environ Toxicol Chem 11:2699–2708. doi:10.1897/03-544
Wheelock CE, Phillips BM, Anderson BS, Miller JL, Miller MJ, Hammock BD (2008) Applications of carboxylesterase activity in environmental monitoring and toxicity identification evaluations (TIEs). Rev Environ Contam Toxicol 195:117–178. doi:10.1007/978-0-387-77030-7_5
Acknowledgments
We wish to thank Mateo R. and Chuiko G. for critical comments and valuable suggestions that improved the manuscript. We thank Elberg G. for help during sample collection and farmers for permission to work in their rice fields. This work was supported by PICT-SECYT No. 1148 (Director: Dr. Rafael C. Lajmanovich).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Attademo, A.M., Cabagna-Zenklusen, M., Lajmanovich, R.C. et al. B-esterase activities and blood cell morphology in the frog Leptodactylus chaquensis (Amphibia: Leptodactylidae) on rice agroecosystems from Santa Fe Province (Argentina). Ecotoxicology 20, 274–282 (2011). https://doi.org/10.1007/s10646-010-0579-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0579-8