Abstract
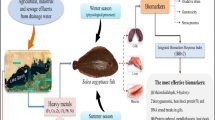
Biomarkers measured at the molecular and cellular level in fish have been proposed as sensitive “early warning” tools for biological effect measurements in environmental quality assessments. Lake Beira is a hypertrophic urban water body with a complex mixture of pollutants including polycyclic aromatic hydrocarbons (PAHs) and Microcystins. In this study, a suite of biomarker responses viz. biliary fluorescent aromatic compounds (FACs), hepatic ethoxyresorufin O-deethylase (EROD) and glutathione S-transferase (GST), brain and muscle cholinesterases (ChE), serum sorbitol dehydrogenase (SDH), and liver histology of Oreochromis niloticus, the dominant fish inhabiting this tropical Lake were evaluated to assess the pollution exposure and biological effects. Some fish sampled in the dry periods demonstrated prominent structural abnormalities in the liver and concomitant increase in serum SDH and reduction in hepatic GST activities in comparison to the control fish and the fish sampled in the rainy periods. The resident fish with apparently normal liver demonstrated induction of hepatic EROD and GST activities and increase in biliary FACs irrespective of the sampling period indicating bioavailability of PAHs. Muscle ChE activities of the resident fish were depressed significantly indicating exposure to anticholinesterase substances. The results revealed that fish populations residing in this Lake is under threat due to the pollution stress. Hepatic abnormalities in the fish may be mainly associated with the pollution stress due to recurrent exposure to PAHs and toxigenic Microcystis blooms in the Lake.




Similar content being viewed by others
References
Aas E, Beyer J, Goksoyr A (2000) Fixed wavelength fluorescence (FF) of bile as a monitoring tool for polyaromatic hydrocarbon exposure in fish: an evaluation of compound specificity, inner filter effect and signal interpretation. Biomarkers 5:9–23
Amado LL, Monserrat JM (2010) Oxidative stress generation by Microcystins in aquatic animals: Why and how. Environ Int 36:226–235
Atencio L, Moreno I, Prieto AI, Moyano R, Molina AM, Camean AM (2008) Acute effects of Microcystins MC-LR and MC-RR on acid and alkaline phosphatase activities and pathological changes in intraperitoneally exposed tilapia fish (Oreochromis sp.). Toxicol Pathol 36:449–458
Au DWT (2004) The application of histocytopathological biomarkers in marine pollution monitoring: a review. Mar Pollut Bull 48:817–834
Davis TW, Berry DL, Boyer GL, Gobler CJ (2009) The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 8:715–725
Dixon DG, Hodson PV, Kaiser KLE (1987) Serum sorbitol dehydrogenase activity as an indicator of chemically induced liver damage in rainbow trout. Environ Toxicol Chem 6:685–696
Ellman GL, Coutney KD, Anders V Jr, Featherstone RM (1961) A new and rapid colourimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:85–95
Fernandes C, Fontainhas-Fernandes A, Rocha E, Salgado MA (2008) Monitoring pollution in Esmoriz-Paramos lagoon, Portugal: liver histological and biochemical effects in Liza saliens. Environ Monit Assess 145:315–322
Figueiredo-Fernandes AM, Fontainhas-Fernandes AA, Monteiro RAF, Reis-Henriques MA, Rocha E (2007) Spatial relationships of the intrahepatic vascular-biliary tracts and associated pancreatic acini of Nile tilapia, Oreochromis niloticus (Teleostei, Cichlidae): a serial section study by light microscopy. Ann Anat 189:17–30
George SG (1994) Enzymology and molecular biology of phase II xenobiotic conjugating enzymes in fish. In: Malins DC, Ostrander GK (eds) Aquatic toxicology: molecular, biochemical and cellular perspective. Lewis Publishers. CRC Press, pp 37–85
Gerlach U (1983) Sorbitol dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Weinheim, pp 112–117
Gupta N, Pant SC, Vijayaraghavan R, Rao PV (2003) Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology 188:285–296
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Jayatissa LP, Silva EIL, McElhiney J, Lawton LA (2006) Occurrence of toxigenic cyanobacterial blooms in freshwaters of Sri Lanka. Syst Appl Microbiol 29(2):156–164
Kamaladasa AI, Jayatunga YNA (2007) Trophic status of the restored South-West and non-restored East Beira Lakes. J Natl Sci Found Sri Lanka 35(1):41–47
Klotz AV, Stegeman JJ, Walsh C (1984) An alternative 7-ethoxyresorufin o-deethylase activity assay; a continuous visible spectrometric method for measurement of cytochrome P-450 monooxygenase activity. Anal Biochem 140:138–145
Lowry H, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Magana-Arachchi DN, Wanigatunge RP, Jeyanandarajah P (2008) Setting up a polymerase chain reaction assay for the detection of toxic cyanobacteria. J Natl Sci Found Sri Lanka 36(3):29–233
Pathiratne KAS, De Silva OCP, Hehemann D, Atkinson I, Wei R (2007) Occurrence and distribution of polycyclic aromatic hydrocarbons (PAHs) in Bolgoda and Beira Lakes, Sri Lanka. Bull Environ Contam Toxicol 79:135–140
Pathiratne A, Chandrasekara LWHU, De Seram PKC (2008) Effects of biological and technical factors on brain and muscle cholinesterases in Nile tilapia, Oreochromis niloticus: implications for biomonitoring neurotoxic contaminations. Arch Environ Contam Toxicol 54:309–317
Pathiratne A, Chandrasekara LWHU, Pathiratne KAS (2009) Use of biomarkers in Nile tilapia (Oreochromis niloticus) to assess the impacts of pollution in Bolgoda Lake, an urban water body in Sri Lanka. Environ Monit Assess 156:361–375
Payne JF, Mathieu A, Melvin W, Fancey LL (1996) Acetylcholinesterase, an old biomarker with a new future? Field trials in association with two urban rivers and a paper mill in New Foundland. Mar Pollut Bull 32:225–231
Roberts RJ (1978) Fish pathology. Bailliere Tindall, London
Sturm A, Wogram J, Segner H, Liess M (2000) Different sensitivity to organophosphates of acetylcholinesterase and butylcholinesterases from three-spined stickleback (Gasterosteus aculeatus): application in biomonitoring. Environ Toxicol Chem 19(6):1607–1615
Treves-Brown KM (2000) Applied fish pharmacology. Kluwer Academic Publishers, Dordrecht, The Netherlands
Van der Oost R, Beyer J, Vermeulan NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Vicentini CA, Franceschini-Vicentini IB, Bombonato MTS, Bertducci B, Lima SG, Santos AS (2005) Morphological study of the liver in the teleost Oreochromis niloticus. Int J Morphol 23(30):211–216
Whyte JJ, Jung RE, Schmitt CJ, Tillitt DE (2000) Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit Rev Toxicol 30:347–570
Yadav A, Gopesh A, Pandey RS, Rai DK, Sharma B (2009) Acetylcholinesterase: a potential biochemical indicator for biomonitoring of fertilizer industry effluent toxicity in freshwater teleost, Channa striatus. Ecotoxicology 18:325–333
Zar JH (1999) Biostatistical analysis. Prentice Hall, Upper Saddle River, NJ
Acknowledgements
We thank Prof. M. D. P. De Costa for granting us permission to use their facilities for fluorescence determinations in bile samples and Mr. D. D. R. U. Wanigesekera, for assistance with the histological preparations. This study was financially supported by National Science Foundation of Sri Lanka (RG/2003/ZOO/05).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pathiratne, A., Pathiratne, K.A.S. & De Seram, P.K.C. Assessment of biological effects of pollutants in a hyper eutrophic tropical water body, Lake Beira, Sri Lanka using multiple biomarker responses of resident fish, Nile tilapia (Oreochromis niloticus). Ecotoxicology 19, 1019–1026 (2010). https://doi.org/10.1007/s10646-010-0483-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0483-2