Abstract
Rising temperatures, drought, and oxygen depletion may be the greatest threats to aquatic animals in the twenty-first century. As a robust body of literature suggests, large-bodied fish are among the most vulnerable organisms in times of rapid climate change. While earlier studies showed an interspecific correlation between body size and sensitivity to hypoxia and thermal stress, comparisons within species remain debated. This review marshals a diverse body of literature on this topic, ranging from physiological studies to field reports and fish kill manuals, and evaluates the evidence for intraspecific size effects on hypoxia tolerance. While experimental studies and fisheries management literature sometimes contradict each other, we show that there is strong evidence for size effects on hypoxia tolerance within fish species. We argue that bringing fisheries management literature and physiological studies into a dialog with each other is of crucial importance in times of rapid climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The relationship between body size and hypoxia in fishes is a point of debate since at least two decades (see, e.g., Nilsson and Östlund-Nilsson 2008; Rogers et al. 2016; 2021; Verberk et al. 2022). In a review of this issue, Nilsson and Östlund-Nilsson (2008) argued that large fish were more likely to survive when exposed to acute hypoxia or high temperatures. This seems counterintuitive from a fisheries management perspective, as it is commonly observed that larger individuals within a population are over-represented in hypoxia- and temperature-induced fish kill events. The over-representation of larger fish in hypoxia- and heat-induced die-off events is widely reported in field reports and informs most instructional manuals for fieldwork procedures after fish kills (see, e.g., Meyer and Barclay 1990; Helfrich and Smith 2009; Whitford 2009; Grant et al. 2014; Knowles et al. 2015, as well as the list of 20 manuals in Table 3).
Indeed, as these manuals often indicate, the size distributions of dead fish allow preliminary inferences on the causes of mass die-off event: while the observation of more small and juvenile fish being killed first are taken as an indication of either pesticidal pollution or toxic algal bloom, dead large fish are interpreted as a symptom oxygen depletion. In their instructional section on “interpreting the scene,” most manuals present a catalog of factors and causes of fish kills that also includes the timing of the observed mortality: as Meyer and Barclay (1990) and most other manuals listed in Table 1 indicate, “(l)arge fish killed first, eventually all sizes” is a typical symptom of exposure to hypoxia, while “(s)mall fish killed” points to toxic chemicals or algal blooms. Other manuals elaborate on the causes for size-related mortality in hypoxia-induced fish kills, e.g., “Small fish usually survive because they have a greater gill size ratio than do larger fish.” (Missouri Department of Conservation 1994); see also U.S. Environmental Protection Agency (1972).
This seeming discrepancy between laboratory studies and fieldwork experience raises the question about the reliability of such field manual instructions and the extent to which they are grounded in scientific consensus and, more widely, about the relationship between practice-based knowledge in fisheries management and experimental physiology. In this review, which is complemented by a small dataset on 3 drought-induced fish kills that occurred during the exceptionally dry summers of 2020 and 2022 in the Netherlands, we evaluate the evidence for intraspecific size-related mortality in hypoxia-induced fish kills. Resolving the apparent discrepancy between laboratory and field observations is urgently needed, as rapid climate change increases periods of drought and extreme heat worldwide. A better understanding of the tolerance of fishes in different size classes is of great importance because size- and age-structured mortality plays a key role in the reproductive future and the survival of the entire population. Bringing different types of literature into a dialog with each other allows for a harmonization of the existing knowledge in respiratory physiology, field ecology, and fisheries management literature. In addition to data on critical oxygen levels and mortality of fishes exposed to hypoxia, we also include literature on behavioral responses to oxygen deficiency, mainly aquatic surface breathing (hereafter ASR), as well as recovery from anaerobic metabolism and lactate concentrations in the body.
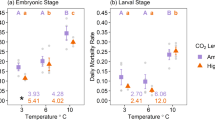
The question of body size in hypoxic environments is at the heart of the current debate about temperature impact on the metabolism and growth of fishes (Hoefnagel and Verberk 2015; Pauly 2019, 2021; Verberk et al. 2021, 2022). Temperature can induce hypoxia in at least two ways: (i) by increasing oxygen demand in ectothermic animals and (ii) by reducing the amount of oxygen that is can be dissolved in warmer water, with the latter factor being far less importance than the former (Fig. 1). Both internal hypoxia and external hypoxia are thus very often a result of increased temperatures, especially during events leading to fish kills.
Above 4–5 °C, the oxygen demand by fish and other water-breathing ectotherms tends to increase with temperature (mainly because of the increasing rate of spontaneous denaturation of proteins), as represented by Krogh’s normal curve (Ege and Krogh 1914). Oxygen solubility only decreases with temperature. Jointly, these two processes, above 4–5 °C, cause fish to suffocate at temperatures higher than those to which they are adapted
Atmospheric oxygen concentrations have been associated with the evolution of different body size in various classes of animals: insect gigantism occurred in geological periods when the oxygen concentrations were significantly higher than in the present—with records of up to 35% during the later Paleozoic (Harrison et al. 2010; Verberk and Bilton 2011; Ward 2006). Similarly, the fossil record provides evidence that the body sizes of fishes, both teleosts and elasmobranchs, declined in warmer periods and increased with more oxygen and lower temperatures (Troyer et al. 2022; Shimada et al. 2022).
In their review paper, titled “Does size matter for hypoxia tolerance in fish?”, Nilsson and Östlund-Nilsson (2008) presented a contrasting perspective. Citing data on 15 fish species from 8 studies, and adding their own findings on damselfish, they argued that “body size per se has little or no impact on the ability to take up oxygen during hypoxic conditions, primarily because the respiratory surface area matches metabolic rate over a wide size range.” However, they concluded that size should indeed matter because larger individuals have a higher capacity for anaerobic ATP production, which allow them to survive longer during severe hypoxia. Under this scenario, given the high levels of lactate that is released as a result of anaerobic metabolism, larger individuals would have an advantage over smaller ones because they would be more resistant to the effects of these waste products.
This observation is indeed confirmed by an extensive body of literature, as large individuals often rely on anaerobic metabolism (Somero and Childress 1980 and the references in the section Body size and anaerobic metabolism), while smaller conspecifics usually do not possess this capacity. Indeed, the need for larger fish to resort to glycolysis (or other forms of anaerobic metabolism) in order to maintain their vital functions is well-documented. Goolish (1991), for example, noted that while smaller fish were able to perform energy-consuming sprints—up to their very physical limits—without using anaerobic resources, larger fish could not perform even moderate physical exercise without glycolysis. Their capacity of oxygen uptake was limited compared to smaller individuals and they depended on an alternative supply to maintain their active metabolism. Nilsson and Östlund-Nilsson (2008), however, turned this observation on its head and concluded that larger fish were therefore not oxygen-limited.
A strong negative correlation between body mass and hypoxia tolerance was found in a recent review by Verberk et al. (2022) which, however, mainly focused on differences between species and on physiological literature. The interspecific size differences of the species in their dataset spanned several orders of magnitude, which made it difficult to tease out differences in ontogeny within species. While this interspecific comparison has invaluable macroecological implications, and may also explain larger biogeographical patterns, intraspecific effects of body size on hypoxia tolerance and thermal sensitivity remain unclear and require a more comprehensive evaluation. In this contribution, we revisit such effects within species by comparing different bodies of literature, from physiological studies based on laboratory data to fieldwork manuals and fish kill reports. We then discuss a number of caveats that should be considered when interpreting available data relating hypoxia to fish size.
Materials and methods
We conducted a literature search in Google Scholar, Science Direct, and the Biodiversity Heritage Library and collected data on three described phenomena: (1) sensitivity to hypoxia and high temperatures, (2) aquatic surface breathing, and (3) size-related scaling of anaerobic metabolism. The latter is relevant, since Nilsson and Östlund-Nilsson (2008) build their argument around the increased anaerobic capacity of large fish. For (1), we only included publications that explicitly mentioned size-related differences in hypoxia tolerances within a given species (both positive and negative effects of size). We excluded studies on air-breathing fishes, as well as on larvae and young hatchlings, and limited our focus to data on juveniles and adults of various sizes. As Doudoroff and Shumway (1970) have argued, data on the hypoxia tolerance of fishes during the first days after hatching are notoriously contradictory and highly vulnerable to disruptive factors that may not directly correlate with high temperatures and hypoxia but result from poor conditions to which young larvae are more prone in general. We also excluded publications that did not directly specify the species being killed but only mentioned a prevalence of killed adults compared to juveniles (e.g., Martin 1989; Mallin et al. 2002), although they also supported the argument that will be made below. In addition, we reviewed 20 fish kill manuals with fieldwork instructions and extracted their statements about size-related mortality during mass die-offs.
In addition to the literature review, we present data on three fish kills that occurred in the hot summers 2020 and 2022 in Brabant, the south of the Netherlands. The summers from 2018 to 2022 were extremely dry, and both groundwater and surface water levels fell to record lows, especially in the southeastern part of the country (Brakkee et al. 2022). During all three die-off events, the water levels of various freshwater bodies fell quickly, in two cases to puddles > 30 cm (see Table 4), and the management intervention was to save surviving fish and to release them in nearby water bodies in accordance with Dutch regulations. Given that the drought had reduced some water bodies to shallow puddles, it was possible to catch most surviving fish and exclude the possibility of large individuals being overlooked.
Only numbers and sizes of survivors were recorded. As Labay and Buzan (1999) have argued, the numbers of smaller dead fish are often underestimated in fish kill investigations, and larger fish are typically overrepresented in the resulting reports (see also Koehn 2004 and King et al. 2012). Despite the limited scope of our observations, their relevance lies in the fact that they do not suffer from this shortcoming because we focused on the survivors and not on the dead fish and could exclude large undiscovered surviving individuals. This approach allowed for a more realistic perspective on the role of size in hypoxia-induced mass die-offs, and we suggest to follow this approach in future surveys (if drought-induced low water levels allow for comprehensive estimates of the fish that actually survived).
Results
Body size and sensitivity to hypoxia and thermal stress
The reviewed literature as well as the new data present robust evidence for a positive correlation between body size and sensitivity to hypoxia and heat stress. In 30 of the 35 species listed in Table 1, body size was a factor that reduced the tolerance of hypoxia and higher temperature. The species for which a higher degree of hypoxia tolerance at greater body sizes was indicated are likely to possess more anaerobic capacity at larger size. Oscar, Astronotus ocellatus (Agassiz, 1831), for example, a cichlid known for its hardiness in hypoxic environments and tolerance of heat stress and oxygen depletion, increases with size because larger fish are more resistant to the resulting waste products of anaerobic metabolism (Almeida-Val et al. 2000). However, larger individuals were still more likely to show aquatic surface breathing, which indicates that they are more oxygen-limited despite their increased capacity to produce ATP anaerobically.
Our results are largely in line with the results of an earlier review by Davis (1975), as far as interspecific size differences are evaluated there (Table 4). We did not use these results, however, because his compilation of data on different fish sizes are from different studies, and the indication of size and temperatures was not always precise enough to allow for direct inferences of the role of size.
Aquatic surface breathing (ASR)
The authors of most studies on ASR reported an increase in surface breathing behavior with increasing body size (Table 2). The exception was Schofield and Chapman (2000) who did not observe a correlation in Lates niloticus (L.), in contrast to Reid et al. (2013). Sukhum et al. (2016) found a positive correlation between aquatic surface breathing and brain size in different species of Osteoglossomorph species, but did not evaluate body mass.
Body size and anaerobic metabolism
The studies by Goolish (1989ab; 1991; 1995), Kieffer (1995; 2000; 2010), Kieffer et al. (1996), Kieffer and Tufts (1998), Ferguson et al. (1993), Gingerich and Suski (2012), Somero and Childress (1980); Zhang et al. (2014), Urbina and Glover (2013), McDonald et al. (1998), Moyes and West (1995), and Clark et al. (2012) on anaerobic metabolism all reported a positive correlation with body size. Urbina and Glover’s (2013) statement on Inanga, Galaxias maculatus (Jenyns, 1842), may apply to more species and perhaps to fish in general: “Owing to their size and higher lactate production rates, larger inanga can accumulate up to 16.6 times more lactate than small fish. Therefore, these data suggest that the capacity for anaerobic metabolism is size dependent and scales with fish size.”
Fish kill manuals
Nineteen out of 20 reviewed fish kill manuals that specify hypoxia-induced die-offs indicate a greater sensitivity to hypoxia in adults compared to juveniles (Table 3). One manual (Dallas and Day 2004) indicates the opposite and a greater vulnerability in juveniles. However, this greater vulnerability appears to apply to the longer-term “secondary” effect of fish kills rather than the short-term deaths caused by hypoxia.
Survival of juvenile fishes in three fish kills in the Netherlands
The three fish kills documented in Table 4 confirm the inference that can be drawn from the literature presented in Table 1: hypoxia and heat stress affect larger fish far more drastically than smaller individuals. Since these data focused on survivors, the counting bias described by Labay and Buzan (1999) and suggested by Koehn (2004) and King et al. (2012) did not affect our results. In all cases, juvenile cyprinids had the greatest probability of survival, while older and larger size classes were often wiped out entirely. In places where sticklebacks occurred naturally, they made up the main portion of survivors—perhaps due to their tolerance of high salinity levels, which are typical at extreme drought events. Pike, perch, and tench only survived as juveniles (all smaller than 10–15 cm). In the case of Omleidingskanaal Groote Beerze, perch were the first to die, and dead large perch were first reported on August 26, 2022, after a short heatwave had driven up local (air) temperatures up to 33.5 °C. The other fish died in the following days when the air temperatures were already lower but the water level had sunk quickly. While drought-induced fish kills may result in a higher vulnerability for larger individuals to avian predators, because they can be easier located in shallow water, this seems unlikely at locations 1 and 2 (Strijpsche Beek), where the fish had access to culverts or where a culvert was the only spot that still held water.
Discussion
The existing literature on hypoxia sensitivity, aquatic surface breathing, and anaerobic metabolism, as well as the data on the survival of juveniles in fish kills, allows for drawing three conclusions:
-
1)
Adult fish, especially larger individuals, are more sensitive to hypoxia and heat stress.
-
2)
Adult fish, especially larger individuals, show more and earlier surface breathing when exposed to hypoxia.
-
3)
Larger fish are more capable of (and dependent on) anaerobic metabolism, which can allow them to resist hypoxia for short periods.
Nineteen out of 20 of the fish kill manuals reviewed here confirmed these findings. If the role of size was mentioned, they indicated a correlation between size/age and the risk of being killed by thermal stress and/or oxygen depletion. However, this does not imply that these manuals necessarily provide correct explanations for this phenomenon. Indeed, field personnel sometimes overestimate the metabolic and oxygen demand of larger fish. As Grant et al. (2014) argue “[…] larger fish are more susceptible to dissolved oxygen depletion due to their higher metabolic requirements relative to smaller individuals, but are often found in higher abundances in the lower leaches of a watercourse.” This explanation is not entirely accurate because oxygen consumption does not scale proportionally to body weight; rather, it shows a negative allometry, which implies a reduction of mass-specific oxygen consumption in larger fish (see, e.g., Schmidt-Nielsen 1984).
However, this imperfect understanding of metabolic scaling is still more in line with empirical observations than the interpretation of data on oxygen demand and supply by Nilsson and Östlund-Nilsson (2008). What both perspectives fail to consider is that while oxygen consumption is indeed reduced with increasing body size, so is the ratio between gill surface area and body mass. In fact, the latter is the cause of the former, i.e., the relative decrease in respiratory surface explains both why larger fish switch to anaerobic metabolism and why they are still more vulnerable to severe hypoxia. While larger organisms may indeed have a short-term advantage due to their anaerobic capacity, this resource is not endless, and after a while, they have to switch back to aerobic metabolism. This explains why large fish may seem more hypoxia-tolerant in short-term laboratory settings and can show enhanced swimming capacity in hypoxic water (Oldham et al. 2019) but are in fact more vulnerable to oxygen depletion than smaller individuals.
Our findings further support the conclusions of Leiva et al. (2019) and Verberk et al. (2022), who inferred correlations between hypoxia tolerance and body size from a wide variety of experimental data but focused on comparisons between species. Given the urgency of increasing drought events and heatwaves in a warming climate, these results require a more general explanation. A mechanism that is a priori excluded by Nilsson and Östlund-Nilsson (2008) is the scaling relationship between respiratory surfaces and the body mass they have to provide with oxygen at different body sizes. As they argue, “oxygen consumption and gill surface area show virtually identical scaling exponents, suggesting that gill surface area is matched with the requirement for oxygen uptake over a large body size range, and that the ability to take up oxygen during hypoxia is independent of size”. While it is obviously correct that oxygen consumption rate and gill surface area scale with similar exponents, the idea that “the ability to take up oxygen during hypoxia is independent of size” needs revision, as suggested by the observation that both anaerobic metabolism and the recovery period increase with body mass. If oxygen uptake capacity was indeed independent of size, larger fish would not have to rely on anaerobic metabolism for even moderate physical activities (see the studies cited above in the section Body size and anaerobic metabolism.
Similar assumptions also underly the arguments in Scheuffele et al. (2021) and Seibel and Deutsch (2020) who contend that the similar (or identical) scaling exponents of oxygen consumption and gill surface area rule out the possibility of oxygen limitation because “as whole-animal metabolic rate increases with size, the oxygen supply capacity increases to match it” (Seibel and Deutsch 2020). This statement overlooks the fact that large adults invest less energy in growth because almost all is needed for maintenance metabolism. If the growth of adults were to continue at the same rate as in juveniles, it is clear that no gills could exist (within the constraints of fish anatomy) with a surface area that could support their growing body with sufficient oxygen. The assumption that the similar scaling exponents of gill surface area and oxygen uptake suggest size-independence of aerobic capacity is only valid if growth expenditure can be excluded from the metabolic rate that is measured. However, this is rarely the case. As Rosenfeld et al. (2015) demonstrate, measured standard metabolic rate (SMR) is “strongly affected by growth rate, that is, elevated metabolism associated with tissue synthesis will affect whole-body metabolism even when organisms are at rest […].” Even after sufficient fasting periods, overhead costs of growth can still play a significant role during respirometry. The authors therefore recommend maintenance rations weeks in advance of the experiments. In the data that are cited in the current literature, this is not the case, which points at a problem that may be specific to fish physiology: “Because of the strong influence of growth on SMR, physiologists studying maintenance metabolism in birds and mammals generally work on adults; in contrast, fish physiologists commonly measure SMR on actively growing juveniles in laboratory experiments […]” (Rosenfeld et al. 2015).
Despite the helpful and now widely adopted suggestions by Chabot et al. (2016), who define a number of guidelines on how to measure standard metabolic rate in fish, the data that are available and cited in the literature so far, do not typically conform to these standards. Following Rosenfeld et al. (2015), Chabot et al. (2016) recommend that “standard metabolic rate” should be defined as metabolic rate excluding growth expenditure (for an overview of debates on definitions of relevant parameters, see also Claireaux and Chabot 2016). However, most of the studies cited in Scheuffele et al. (2021), Seibel and Deutsch (2020), or Lefevre et al. (2017) do not even indicate that they measure standard metabolic rate but often refer to resting metabolic rate (RMR). Studies that indicate the exact protocol according to which fasting periods and maintenance rations were determined are still rare (but see, e.g., Tirsgaard et al. 2015). The interspecific exponents of either SMR and RMR that are reported in the existing literature vary between 0.79 and 0.94 (see, e.g., Clarke and Johnston 1999; Killen et al. 2007; Lefevre et al. 2017; Jerde et al. 2019; Ye et al. 2021; Harter et al. 2022).
The observation that smaller and younger fish have a larger aerobic scope (which allows them to grow but also to compensate for a temperature-induced increase in energetic costs) is consistent with, and can be coherently explained by, the Gill-Oxygen Limitation Theory (GOLT) as developed by Pauly (197920192021). According to the GOLT, gill surface area is a limiting factor for fish growth because gills, as two-dimensional surfaces, have to support three-dimensional bodies, even if they grow with positive allometry. While fish growth slows down once the respiratory surfaces supply the body with relatively less oxygen, a drastic increase in temperature and critical levels of hypoxia are more difficult to cope with for individuals that are already near the limit of their capacity to supply the body with oxygen (Fig. 2).
This is especially the case in fishes that have grown to larger sizes in cooler environments. At higher temperatures, fish do typically not reach the maximum sizes that would be possible for their species (see, e.g., Dimarchopoulou and Tsikliras 2022). The GOLT explains this difference in growth as a result of the difference between anabolism and catabolism, i.e.,
where dw/dt is the growth rate, HWd is the rate of protein synthesis, which requires oxygen, and kW is the rate at which proteins spontaneously denature, a process that does not require oxygen itself but that implies the need to re-synthesize and replace the denatured proteins (see Pauly 2019, 2021; Pauly and Lam 2023). The parameter d, which in fish, ranges from 0.6 to 0.9, is the exponent in the relationship linking the surface area (S) of the gills to body weight (W), such that S ∝ Wd. As shown by Scheuffele et al. (2021), d tends to be equal or very close to the scaling exponent of measured oxygen uptake (which is often not the same as “standard metabolic rate” in juvenile and growing fish, if we follow the definition of Chabot et al. 2016). Since kW increases with W, it will gradually approach HWd, which will cause growth to slow down and stop (when HWd = kW). Thus, if temperature increases protein denaturation and thus kW (which it does, see Fig. 2), HWd = kW is reached at smaller sizes.
This phenomenon, which is often described as the “temperature-size rule,” can then be explained as a physiological mechanism that occurs in individual ontogenies. In environments where heat stress occurs, this mechanism may also have an adaptive advantage: if fish remain small, they may be better able to survive in hot and hypoxic environments. Reduced final sizes at higher temperatures can then be regarded as “[b]enefits of being small in a changing world” (Clark et al. 2012)—benefits that do not come without a cost, for example, fewer and smaller eggs (Hislop 1988). For the thermal and hypoxia tolerance of individuals, however, the advantage of smallness in hypoxic and hyperthermic conditions can be explained as a result of the relationship between respiratory surfaces and body volume, even when the scaling exponents of gill surface area and oxygen uptake are similar or equal. As Blasco et al. (2022) argue, even if “AS [aerobic scope] scales isometrically with body mass across multiple species,” this only applies within a limited thermal range. With increasing temperatures, larger animal are more oxygen-limited because “gill functional respiratory surface area declines with mass in fishes.” It should be noted that many fish species can alter their gill surface area as a response to higher demand, for example, during hypoxia or hyperthermia. However, the capacity to increase their respiratory surfaces is only very limited (or even absent) in marine species, and its efficiency in freshwater fish is impeded by the resulting ion loss that would be a result of larger gills (Bowden et al. 2014; Wood and Eom 2021). According to Bowden et al. (2014), it is therefore unclear if such forms of gill plasticity (or gill remodeling) will allow for sufficient adaptation, especially under current climate predictions. In the case of hypoxic events or heatwaves, gill remodeling is also unlikely to be effective as it requires time and such events often occur quickly.
A possible hypothesis that could be further examined in future studies would be that the size effects discussed above may depend on the combined effect of hypoxia and thermal stress. This would also explain why studies on winter fish kills do not report strong size effects or even show an advantage of larger adults over smaller individuals or juveniles (see, e.g., Davis et al. 2019). According to our model, it is possible that temperature may have a stronger impact on size-related oxygen limitation than hypoxia, since hypoxia does not increase maintenance metabolism. In summer fish kills, the effects of the two factors are difficult to tease apart because both thermal and hypoxic stress contribute to internal hypoxia in fish.
Literature on aquatic invertebrates suggests that our observations allow for generalizations that extend oxygen limitation in fishes only and that they can be applied to other phyla. Data on molluscs, and especially clams, report the same pattern: larger animal rely more heavily on anaerobic metabolism, but are nonetheless the first to die in hypoxic environments and under heat stress (Clark et al. 2013; Cueto-Vega et al. 2022; Aguirre-Velarde et al. 2019; Garlo et al. 1979). Clark et al. (2013) generalize this as follows: “… older animals have a proportionally smaller gill surface for oxygen extraction. Older animals have a lower metabolic rate, but tissue scaling probably contributes towards the age-related stress effects seen in older animals.” This mechanism may eventually apply to all water-breathing organisms but more research is needed here (for a comparison between different phyla, see Table 2 in Pauly et al. 2022).
As J.B.S. Haldane (1926) elegantly put it in his seminal essay On Being the Right Size, “Comparative anatomy is largely the story of the struggle to increase surface in proportion to volume.” The struggle for surface in relation to volume also underlies the survival of water-breathing animals in times of rapid climate change, and it is their adaptability to new thermal environments that will decide their fate in a warming world.
Data availability
All data generated or analyzed during this study are included in this published article. More documentation can be provided at request.
References
Abdallah SJ, Thomas BS, Jonz MG (2015) Aquatic surface respiration and swimming behaviour in adult and developing zebrafish exposed to hypoxia. J Exp Biol 218(11):1777–1786
Aguirre-Velarde A, Thouzeau G, Jean F, Mendo J, Cueto-Vega R, Kawazo-Delgado M, Vásquez-Spencer J, Herrera-Sanchez D, Vega-Espinoza A, Flye-Sainte-Marie J (2019) Chronic and severe hypoxic conditions in Paracas Bay, Pisco, Peru: consequences on scallop growth, reproduction, and survival. Aquaculture 512:734259
Almeida-Val VM, Val AL, Duncan WP, Souza FC, Paula-Silva MN, Land S (2000) Scaling effects on hypoxia tolerance in the Amazon fish Astronotus ocellatus (Perciformes: Cichlidae): contribution of tissue enzyme levels. Comp Biochem Physiol Part B: Biochem Mol Biol 125(2):219–226
American University of Beirut (2013) Litani river basin management support. Water quality index. U.S. Agency for International Development, Washington
Bailey RM (1955) Differential mortality from high temperatures in a mixed population of fishes in southern Michigan. Ecology 36:526–528
Bauch G (1949) Untersuchungen über das Wachstum der Kleinen Maräne (Coregonus albula) in den Gewässern Mitteleuropas. Abhandl Fisch Hilfswiss 2:252–326
Blasco FR, Taylor EW, Leite CA, Monteiro DA, Rantin FT, McKenzie DJ (2022) Tolerance of an acute warming challenge declines with body mass in Nile tilapia: evidence of a link to capacity for oxygen uptake. J Exp Biol 225(16):jeb244287
Bowden AJ, Gardiner NM, Couturier CS, Stecyk JAW, Nilsson GE, Munday PL, Rummer JL (2014) Alterations in gill structure in tropical reef fishes as a result of elevated temperatures. Comp Biochem Physiol Part A Mol Integr Physiol 175:64–71
Brakkee E, Van Huijgevoort MH, Bartholomeus RP (2022) Improved understanding of regional groundwater drought development through time series modelling: the 2018–2019 drought in the Netherlands. Hydrol Earth Syst Sci 26(3):551–569
Burleson ML, Wilhelm DR, Smatresk NJ (2001) The influence of fish size on the avoidance of hypoxia and oxygen selection by largemouth bass. J Fish Biol 59(5):1336–1349
Chabot D, Steffensen JF, Farrell AP (2016) The determination of the standard metabolic rate in fishes. J Fish Biol 88:81–121
Claireaux G, Chabot D (2016) Responses by fishes to environmental hypoxia: integration through Fry’s concept of aerobic metabolic scope. J Fish Biol 88:232–251
Clark TD, Sandblom E, Cox GK, Hinch SG, Farrell AP (2008) Circulatory limits to oxygen supply during an acute temperature increase in the Chinook salmon (Oncorhynchus tshawytscha). Am J Phys - Reg Integr Comp Phys 295:R1631–R1639
Clark TD, Donaldson MR, Pieperhoff S, Drenner SM, Lotto A, Cooke SJ, Hinch SG, Patterson DA, Farrell AP (2012) Physiological benefits of being small in a changing world: responses of Coho salmon (Oncorhynchus kisutch) to an acute thermal challenge and a simulated capture event. PLoS ONE 7(6):e39079
Clark MS, Husmann G, Thorne MA, Burns G, Truebano M, Peck LS, Abele D, Philipp EE (2013) Hypoxia impacts large adults first: consequences in a warming world. Glob Change Biol 19:2251–2263
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905
Colby PJ, Brooke LT (1969) Cisco (Coregonus artedii) mortalities in a southern Michigan lake, July 1968. Limn Oceanogr 14:958–960
Cueto-Vega R, Flye-Sainte-Marie J, Aguirre-Velarde A, Jean F, Gil-Kodaka P, Thouzeau G (2022) Size-based survival of cultured Argopecten purpuratus (L, 1819) under severe hypoxia. J World Aquac Soc 53(1):151–173
Dallas HF, Day JA (2004) The effect of water quality variables on aquatic ecosystems: a review. WRC Report No. TT 224/04. Water Res Comm, Pretoria (SA)
Danks M, Rantala HM, Shen L, Sisler SP, Stevens AG (2017) Investigation of fish kills in Minnesota waters, Minnesota Dept. of Natural Resources, St. Paul
Davis JC (1975) Minimal dissolved oxygen requirements of aquatic life with emphasis on Canadian species: a review. J Fish Board Can 32(12):2295–2332
Davis MN, McMahon TE, Webb MAH, Ilgen JE, Hitch AT, Jaeger ME, Cutting KA (2019) Winter survival, habitat use, and hypoxia tolerance of Montana Arctic Grayling in a winterkill-prone lake. Trans Am Fish Soc 148:843–856
Di Santo V, Lobel PS (2017) Body size and thermal tolerance in tropical gobies. J Exp Mar Biol Ecol 487:11–17
Dimarchopoulou D, Tsikliras AC (2022) Linking growth patterns to sea temperature and oxygen levels across European sardine (Sardina pilchardus) populations. Environ Biol Fish 105(10):1335–1345. https://doi.org/10.1007/s10641-022-01229-5
Doudoroff P, Shumway DL (1970) Dissolved oxygen requirements of freshwater fishes. FAO Technical Paper 86. FAO, Rome
Edsall TA, Colby PJ (1970) Temperature tolerance of young-of-the-year cisco, Coregonus artedii. Trans Am Fish Soc 69:526–531
Ege R, Krogh A (1914) On the relation between the temperature and the respiratory exchange in fishes. Inter Rev Hydrobiol Hydrogr 7:48–55. https://doi.org/10.1002/iroh.19140070105
Ferguson RA, Kieffer JD, Tufts BL (1993) The effects of body size on the acid-base and metabolite status in the white muscle of rainbow trout before and after exhaustive exercise. J Exp Biol 180(1):195–207
Fish GR (1956) Some aspects of the respiration of six species of fish from Uganda. J Exp Biol 33(1):186–195
Francis-Floyd R (1997) Dissolved oxygen for fish production. University of Florida Cooperative Extension Service, Institute of Food and Agriculture Sciences, EDIS, Gainesville/FL
Francis-Floyd R, Riggs A, Philips E (2004) A beginner’s guide to water management–fish kills. EDIS, Gainesville/FL
Frey DG (1955) Distributional ecology of the cisco, Coregonus artedii. Invest Indiana Lakes Streams 4:177–228
Fry FE (1937) The summer migration of the cisco (Leucichthys artedi) (LeSueur), in Lake Nippissing. University of Toronto Press, Ontario
Garlo EV, Milstein CB, Jahn AE (1979) Impact of hypoxic conditions in the vicinity of Little Egg Inlet, New Jersey in summer 1976. Estuar Coast Mar Sci 8(5):421–432
Gingerich AJ, Suski CD (2012) The effect of body size on post-exercise physiology in largemouth bass. Fish Phys Biochem 38(2):329–340
Goolish EM (1989a) A comparison of oxygen debt in small and large rainbow trout, Salmo gairdneri Richardson. J Fish Biol 35:597–598
Goolish EM (1989b) The scaling of aerobic and anaerobic muscle power in rainbow trout (Salmo gairdneri). J Exp Biol 147(1):493–505
Goolish EM (1991) Aerobic and anaerobic scaling in fish. Biol Rev 66:33–56
Goolish EM (1995) The metabolic consequences of body size. In: Hochachka PW, Mommsen TP (eds) Biochemistry and molecular biology of fishes, vol 4. Elsevier, Amsterdam, pp 335–366
Grant B, Huchzermeyer D, Hohls B (2014) Manual for fish kill investigations in South Africa. Report for the Water Research Commission. WRC Rep No. TT, 589(14)
Haldane JB (1926) On being the right size. Harp Mag 152:424–427
Harrison JF, Kaiser A, VandenBrooks JM (2010) Atmospheric oxygen level and the evolution of insect body size. Proceed Roy Soc B: Biol Sci 277(1690):1937–1946
Hart JS (1952) Geographic variations of some physiological and morphological characters in certain freshwater fish. University of Toronto Biology Series no. 60. University of Toronto Press, Toronto
Harter TS, Damsgaard C, Regan MD (2022) Linking environmental salinity to respiratory phenotypes and metabolic rate in fishes: a data mining and modelling approach. J Exp Biol 225:jeb243421
Helfrich LA, Smith SA (2009) Fish kills: their causes and prevention. College of Agriculture and Life Sciences, Virginia Polytechnic Institute and State University, Blacksburg
Hislop JRG (1988) The influence of maternal length and age on the size and weight of the eggs and the relative fecundity of the haddock, Melanogrammus aeglefinus, in British waters. J Fish Biol 32(6):923–930
Hoefnagel KN, Verberk WC (2015) Is the temperature-size rule mediated by oxygen in aquatic ectotherms? J Therm Biol 54:56–65
Huntsman AG (1942) (1942) Death of salmon and trout with high temperature. J Fish Board Can 5(5):485–501
Jacobson PC, Jones TS, Rivers P, Pereira DL (2008) Field estimation of a lethal oxythermal niche boundary for adult ciscoes in Minnesota lakes. Trans Am Fish Soc 137(5):1464–1474
Jerde CL, Kraskura K, Eliason EJ, Csik SR, Stier AC, Taper ML (2019) Strong evidence for an intraspecific metabolic scaling coefficient near 0.89 in fish. Front Physiol 10:1166
Kalinin AL, Rantin FT, Glass ML (1993) Dependence on body size of respiratory function in Hoplias malabaricus during graded hypoxia. Fish Phys Biochem 12:47–51
Kieffer JD (1995) The role of body size and temperature in the physiological response to exercise in fish. PhD. Thesis, Queen’s University, Kingston, ON
Kieffer JD, Ferguson RA, Tompa JE, Tufts BL (1996) Relationship between body size and anaerobic metabolism in brook trout and largemouth bass. Trans Am Fish Soc 125(5):760–767
Kieffer JD, Tufts BL (1998) Effects of food deprivation on white muscle energy reserves in rainbow trout (Oncorhynchus mykiss): the relationships with body size and temperature. Fish Phys Biochem 19(3):239–245
Kieffer JD (2000) Limits to exhaustive exercise in fish. Comp Biochem Physiol Part A Mol Integr Physiol 126(2):161–179
Kieffer JD (2010) Perspective—exercise in fish: 50+ years and going strong. Comp Biochem Physiol Part A Mol Integr Physiol 156(2):163–168
Killen SS, Costa I, Brown JA, Gamperl AK (2007) Little left in the tank: metabolic scaling in marine teleosts and its implications for aerobic scope. Proc Royal Soc B, Biol Sci 274(1608):431–438
King AJ, Tonkin Z, Lieshcke J (2012) Short-term effects of a prolonged blackwater event on aquatic fauna in the Murray River, Australia: considerations for future events. Mar Freshw Res 63:576–586
Knowles SJ, Massarani S, Phelps N (2015) Minnesota fish kill investigation manual. University of Minnesota, College of Veterinary Medicine, Saint Paul
Koehn JD (2004) The loss of valuable Murray cod in fish kills: a science and management perspective. In: Lintermans M, Phillips B (eds) Management of Murray cod in the Murray-Darling Basin. Murray-Darling Basin Comm, Canberra
Komoroske LM, Connon RE, Lindberg J, Cheng BS, Castillo G, Hasenbein M, Fangue NA (2014) Ontogeny influences sensitivity to climate change stressors in an endangered fish. Conserv Physiol 2(1):cou008
Kramer DL (1983) Aquatic surface respiration in the fishes of Panama: distribution in relation to risk of hypoxia. Environ Biol Fish 8:49–54
Kramer DL, Mehegan JP (1981) Aquatic surface respiration, an adaptive response to hypoxia in the guppy, Poecilia reticulata (Pisces, Poeciliidae). Environ Biol Fish 6(3):299–313
Krauch HC (1982)Fish kills in Indiana-their causes and prevention. Wildlife and Fisheries, Cooperative Extension Services, Purdue University, West Lafayette https://www.extension.purdue.edu/extmedia/FNR/FNR-69-W.pdf. Accessed 20 June 2023
Labay AA, Buzan D (1999) A comparison of fish kill counting procedures on a small, narrow stream. North Am J Fish Manag 19(1):209–214
Lefevre S, McKenzie DJ, Nilsson GE (2017) Models projecting the fate of fish populations under climate change need to be based on valid physiological mechanisms. Glob Change Biol 23(9):3449–3459
Leiva FP, Calosi P, Verberk WCEP (2019) Scaling of thermal tolerance with body mass and genome size in ectotherms: a comparison between water- and air-breathers. Phil Trans Roy Soc B: Biol Sci 374(1778):20190035
Lutz CG, Grodner ML (1992) Understanding fish kills. Louisiana Cooperation Extension Service, Breaux Bridge
Mallin MA, Posey MH, McIver MR, Parsons DC, Ensign SH, Alphin TD (2002) Impacts and recovery from multiple hurricanes in a piedmont–coastal plain river system: human development of floodplains greatly compounds the impacts of hurricanes on water quality and aquatic life. Bioscience 52(11):999–1010
Martin KC (1989) A fish kill on the Katherine River, November 1987. North Territory Nat 11:20–26
Matthews WJ (1985) Summer mortality of striped bass in reservoirs of the United States. Trans Am Fish Soc 114(1):62–66
McDonald DG, McFarlane WJ, Milligan CL (1998) Anaerobic capacity and swim performance of juvenile salmonids. Can J Fish Aquat Sci 55(5):1198–1207
Messmer V, Pratchett MS, Hoey AS, Tobin AJ, Coker DJ, Cooke SJ, Clark TD (2017) Global warming may disproportionately affect larger adults in a predatory coral reef fish. Glob Change Biol 23(6):2230–2240
Meyer FP, Barclay LA (1990) Field manual for the investigation of fish kills (No. 177). US Department of the Interior, Fish and Wildlife Service, Bailey’s Crossroads
Mississippi Department of Wildlife, Fisheries, and Parks (2012) Fish kill and sick fish. Mississippi Dept. Wildlife, Fisheries, and Parks, Jackson/MS
Missouri Department of Conservation (1994) Fish kills in ponds and lakes. Missouri Dep Cons, Jefferson City MO. https://mdc.mo.gov/sites/default/files/2020-06/fishkills.pdf. Accessed 20 June 2023
Moyes CD, West TG (1995) Exercise metabolism of fish. In: Hochachka PW, Mommsen TP (eds) Biochemistry and molecular biology of fishes, vol 4. Elsevier, Amsterdam, pp 367–392
New South Wales Department of Primary Industries (2022) Fish kills in NSW. Frequently asked questions. New South Wales Department of Primary Industries, Orange. https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0007/634570/Fish-Kills-FAQ-August-2011.pdf. Accessed 20 June 2023
Nilsson GE, Östlund-Nilsson S (2008) Does size matter for hypoxia tolerance in fish? Biol Rev 83(2):173–189
Oldham T, Nowak B, Hvas M, Oppedal F (2019) Metabolic and functional impacts of hypoxia vary with size in Atlantic salmon. Comp Biochem Physiol Part A Mol Integr Physiol 231:30–38
Ouellet V, Mingelbier M, Saint-Hilaire A, Morin J (2010) Frequency analysis as a tool for assessing adverse conditions during a massive fish kill in the St. Lawrence River, Canada. Water Qual Res J 45(1):47–57
Pan YK, Ern R, Esbaugh AJ (2016) Hypoxia tolerance decreases with body size in red drum Sciaenops ocellatus. J Fish Biol 89(2):1488–1493
Pauly D (1979) Gill size and temperature as governing factors in fish growth: a generalization of von Bertalanffy’s growth formula. Ber Inst Meer Univ Kiel 63
Pauly D (2019) Gasping fish and panting squids: oxygen, temperature and the growth of water-breathing animals – 2nd edition. Excellence in Ecology (22), International Ecology Institute, Oldendorf/Luhe
Pauly D (2021) The gill-oxygen limitation theory (GOLT) and its critics. Sci Adv 7(2):eabc6050
Pauly D, Amarasinghe US, Chu E, Freire KM, Vázquez E, Butler MJ IV (2022) The growth, respiration, and reproduction of crustaceans: a synthesis through the Gill-Oxygen Limitation Theory (GOLT). J Crust Biol 42:1–13. https://doi.org/10.1093/jcbiol/ruac059[witherratum]
Pauly D, Lam ME (2023) Too hot or too cold: the biochemical basis of temperature-size rules for fish and other ectotherms. Environ Biol Fish 106. https://doi.org/10.1007/s10641-023-01429-7
Perkins DL, Kann J, Scoppettone GG (2000) The role of poor water quality and fish kills in the decline of endangered Lost River and shortnose suckers in Upper Klamath Lake. US Geol Surv, Reston
Plath M, Riesch R, Culumber Z, Streit B, Tobler M (2011) Giant water bug (Belostoma sp.) predation on a cave fish (Poecilia mexicana): effects of female body size and gestational state. Evol Ecol Res 13(2):133–144
Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315(5808):95–97
Poukish CA, Driscol CP (1999) Field sampling and necropsy examination of fish. Virg J Sci 50(4):354–364
Ramachandra TV, Sincy V, Asulabha KS, Sudarshan B, Rahaman M F (2016) Recurring fish mortality episodes in Bangalore lakes: sign of irresponsible and fragmented governance. ENVIS Technical Report 105
Rees BB, Matute LA (2018) Repeatable interindividual variation in hypoxia tolerance in the Gulf killifish. Fundulus Grandis Physiol Biochem Zool 91(5):1046–1056
Reid AJ, Farrell MJ, Luke MN, Chapman LJ (2013) Implications of hypoxia tolerance for wetland refugia use in Lake Nabugabo. Uganda Ecol Freshw Fish 22(3):421–429
Robb T, Abrahams MV (2003) Variation in tolerance to hypoxia in a predator and prey species: an ecological advantage of being small? J Fish Biol 62(5):1067–1081
Rogers NJ, Urbina MA, Reardon EE, McKenzie DJ, Wilson RW (2016) A new analysis of hypoxia tolerance in fishes using a database of critical oxygen level (Pcrit). Conserv Phys 4(1):cow012
Rogers NJ, Urbina MA, Reardon EE, McKenzie DJ, Birchenough SN, Wilson RW (2021) Database of critical oxygen level (Pcrit) in freshwater and marine fishes 1974—2015. Centre for Environment, Fisheries and Aquaculture Science. https://www.cefas.co.uk/data-and-publications/dois/database-of-critical-oxygenlevel-pcrit-in-freshwater-and-marine-fishes-1974-2015/. Accessed 20 June 2023
Rosenfeld J, Van Leeuwen T, Richards J, Allen D (2015) Relationship between growth and standard metabolic rate: measurement artefacts and implications for habitat use and life‐history adaptation in salmonids. J Anim Ecol 84(1): 4–20
Scheuffele H, Jutfelt F, Clark TD (2021) Investigating the gill-oxygen limitation hypothesis in fishes: intraspecific scaling relationships of metabolic rate and gill surface area. Conserv Physiol 9(1):coab040
Schmidt-Nielsen K (1984) Scaling: why is animal size so important? Cambridge University Press, Cambridge
Schofield PJ, Chapman LJ (2000) Hypoxia tolerance of introduced Nile perch: implications for survival of indigenous fishes in the Lake Victoria basin. Afr Zool 35(1):35–42
Seibel BA, Deutsch C (2020) Oxygen supply capacity in animals evolves to meet maximum demand at the current oxygen partial pressure regardless of size or temperature. J Exp Biol 23(12):jeb.210492
Shimada K, Maisch HM IV, Perez VJ, Becker MA, Griffiths ML (2022) Revisiting body size trends and nursery areas of the Neogene megatooth shark, Otodus megalodon (Lamniformes: Otodontidae), reveals Bergmann’s rule possibly enhanced its gigantism in cooler waters. Histor Biol 35(2):208–217
Sloman KA, Wood CM, Scott GR, Wood S, Kajimura M, Johannsson OE, Almeida-Val VM, Val AL (2006) Tribute to RG Boutilier: the effect of size on the physiological and behavioural responses of oscar, Astronotus ocellatus, to hypoxia. J Exp Biol 209(7):1197–1205
Sloman KA, Mandic M, Todgham AE, Fangue NA, Subrt P, Richards JG (2008) The response of the tidepool sculpin, Oligocottus maculosus, to hypoxia in laboratory, mesocosm and field environments. Comp Biochem Physiol Part A Mol Integr Physiol 149(3):284–292
Soares MG, Menezes NA, Junk WJ (2006) Adaptations of fish species to oxygen depletion in a central Amazonian floodplain lake. Hydrobiol 568:353–367
Somero GN, Childress JJ (1980) A violation of the metabolism-size scaling paradigm: activities of glycolytic enzymes in muscle increase in larger-size fish. Phys Zool 53(3):322–337
Sukhum KV, Freiler MK, Wang R, Carlson BA (2016) The costs of a big brain: extreme encephalization results in higher energetic demand and reduced hypoxia tolerance in weakly electric African fishes. Proceed Royal Soc B: Biol Sci 283:20162157
Texas Parks and Wildlife Department (1970). Technical series - Texas parks and wildlife, Issue 2. Tex Parks Wildl Dep, Austin
Tirsgaard B, Behrens JW, Steffensen JF (2015) The effect of temperature and body size on metabolic scope of activity in juvenile Atlantic cod Gadus morhua L. Comp Biochem Physiol Part A Mol Integr Physiol 179:89–94
Troyer EM, Betancur-R R, Hughes LC, Westneat M, Carnevale G, White WT, Pogonoski JJ, Tyler JC, Baldwin CC, Ortí G, Brinkworth A (2022) The impact of paleoclimatic changes on body size evolution in marine fishes. Proc Natl Acad Sci U S A 119(29):e2122486119
U.S. Environmental Protection Agency (1972) Investigation of fish kills. Oklahoma Cooperative Fishery Unit, Stillwater
Underwood ZE, Myrick CA, Rogers KB (2012) Effect of acclimation temperature on the upper thermal tolerance of Colorado River cutthroat trout Oncorhynchus clarkii pleuriticus: thermal limits of a North American salmonid. J Fish Biol 80(7):2420–2433
Urbina MA, Glover CN (2013) Relationship between fish size and metabolic rate in the oxyconforming inanga Galaxias maculatus reveals size-dependent strategies to withstand hypoxia. Physiol Biochem Zool 86(6):740–749
Verberk WC, Bilton DT (2011) Can oxygen set thermal limits in an insect and drive gigantism? PLoS ONE 6(7):e22610
Verberk WC, Atkinson D, Hoefnagel KN, Hirst AG, Horne CR, Siepel H (2021) Shrinking body sizes in response to warming: explanations for the temperature–size rule with special emphasis on the role of oxygen. Biol Rev 96(1):247–268
Verberk WC, Sandker JF, van de Pol IL, Urbina MA, Wilson RW, McKenzie DJ, Leiva FP (2022) Body mass and cell size shape the tolerance of fishes to low oxygen in a temperature-dependent manner. Glob Change Biol 28(19):5695–5707
Ward P (2006) Out of thin air: dinosaurs, birds, and earth’s ancient atmosphere. Joseph Henry Press, Washington. https://doi.org/10.17226/11630
Whitford F (2009) What killed the fish? Using observation, sampling, and science to solve the mystery. Purdue University, West Lafayette
Wickman A (1980) The Minnesota forest and wildlife management digest, 2nd ed. Forestry Farmers of Minnesota, Inc. Park Rapids/MN
Willer A (1929) Neue Biologische Beobachtungen über die Kleine Maräne (Coregonus albula L.). Z Fisch 27:251–276
Wood CM, Eom J (2021) The osmorespiratory compromise in the fish gill. Comp Biochem Physiol Part A Mol Integr Physiol 254:110895
Ye X, Lu L, Jiang M, Jia J, Li W, Wu H, Liao Y, Li J (2021) Metabolic scaling: individual versus intraspecific scaling of Nile tilapia (Oreochromis niloticus). J Comp Physiol B Biochem Syst Environ Physiol 191:721–729
Zhang Y, Huang Q, Liu S, He D, Wie G, Luo Y (2014) Intraspecific mass scaling of metabolic rates in grass carp (Ctenopharyngodon idellus). J Comp Physiol B Biochem Syst Environ Physiol 184(3):347–354
Author information
Authors and Affiliations
Contributions
Idea generation: J.M., N.H., and D.P.; case studies: J.M., N.H., and D.P.; data generation for case 5: N.H.; first draft of manuscript: J.M.; editing of final manuscript (and an earlier draft): D.P. and N.H.
Corresponding author
Ethics declarations
Ethical approval
Not applicable. The animals from which the data for case 5 were used were captured (and released) as part of a drought rescue intervention in accordance with the Dutch Uitvoeringsregeling visserij, hoofdstuk 1, § 60a, and for which Sportvisserij Zuidwest Nederland was commissioned.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Müller, J., Houben, N. & Pauly, D. On being the wrong size, or the role of body mass in fish kills and hypoxia exposure. Environ Biol Fish 106, 1651–1667 (2023). https://doi.org/10.1007/s10641-023-01442-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01442-w