Abstract
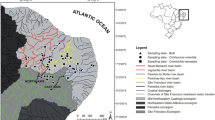
The cichlid Mesonauta festivus is common and abundant among macrophyte stands along a large geographical range of the Amazonas and Paraná-Paraguay basins, in South America. This broad geographical range highlights the species’ dispersion ability, which can be attributed to specific biological and behavioral traits. However, the dispersion ability does not account for the broad geographical range alone, as the species must be able to establish populations in a range of environments, which include marginal areas of large rivers with different water types, floodplain lakes, and small terra-firme streams. In this work we investigated the specie’s ecology, biological traits and behavior in order to understand what and how its traits may have allowed it to attain such broad geographical range and aid in establishing local populations. Regarding its dispersion ability we stress the capability of swimming in the pelagic region, which is remarkable for this species and uncommon among Neotropical cichlids. Its vagility is high even when juveniles are under parental care. Regarding population establishment, the high environmental tolerance stands out, allowing the species to live under strikingly different abiotic conditions. In addition, the small size of first sexual maturation and its capability of spawning along the whole hydrologic cycle (apparently not associated to a specific environmental cue) may also facilitate the establishment of populations into new environments. Moreover, the behavior of mimicking dead leaves, which is mainly performed by juveniles, may lessen predation pressures. Under an eco-evolutionary perspective, the traits highlighted in this work may buffer selective pressures experienced by populations in different biotic and/or abiotic conditions, which may also favor the increasing of the geographical range by allowing the evolutionary lineage to remain similar even in disconnected and/or striking different environments.






Similar content being viewed by others
References
Agostinho AA, Pelicice FM, Júlio HF Jr (2005) Introdução de espécies de peixes em águas continentais brasileiras: Uma síntese. In: Rocha O (ed) Espécies invasoras em águas doces—estudo de caso e propostas de manejo. Editora Universidade Federal de São Carlos, São Carlos, pp 11–23
Almeida-Val VMF, Farias IP, Silva MNP, Duncan WP (1995) Biochemical adjustments to hypoxia in Amazon cichlids. Braz J Med Biochem Res 28:1257–1263
Bates AE, McKelvie CM, Sorte CJ, Morley SA, Jones NA, Mondon JA, Bird TJ, Quinn G (2013) Geographical range, heat tolerance and invasion success in aquatic species. Proc R Soc B 280:20131958
Bradbury IR, Laurel B, Snelgrove PVR, Bentzen P, Campana SE (2008) Global patterns in marine dispersal estimates: the influence of geography, taxonomic category and life history. Proc R Soc B 275:1803–1809
Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK (2011) A standardized terminology for describing reproductive development in fishes. Mar Coast Fish: Dyn, Manag, Ecosyst Sci 3:52–70
Byers JE (2000) Competition between two estuarine snails: implications for invasion of exotic species. Ecol 81:1225–1239
Chippari-Gomes AR, Gomes LC, Lopes NP, Val AL, Almeida-Val V (2005) Metabolic adjustments in two Amazonian cichlids exposed to hypoxia and anoxia. J Comp Physiol B 141:347–355
Costa DI, Romagnoli CF, Carmo LLT, Ribas C, Leite GR, Zuanon J (2011) Ictiofauna associada a bancos de herbáceas aquáticas flutuantes na ilha da Marchantaria, rio Solimões, Amazônia Central, Brasil. Rev Colomb Cienc Anim 3(1):148–156
Crampton WGR (2008) Ecology and life history of an Amazon floodplain cichlid: the discus fish Symphysodon (Perciformes: Cichlidae). Neotrop Ichthyol 6(4):599–612
de Almeida FF, Melo S (2009) Considerações limnológicas sobre um lago da planície de inundação amazônica (lago Catalão—Estado do Amazonas, Brasil). Acta Sci 31(4):387–395
Dias MS, Toledo JJ, Jardim MM, Figueiredo FOGD, Cordeiro CLDO, Gomes ACS, Zuanon J (2011) Congruence between fish and plant assemblages in drifting macrophyte rafts in Central Amazonia. Hydrobiol 661(1):457–461
Drake JM (2007) Parental investment and fecundity, but not brain size, are associated with establishment success in introduced fishes. Funct Ecol 21:963–968
Duponchelle F, Lino F, Hubert N, Panfili J, Renno JF, Baras E, Torrico JP, Dugué R, Nuñez J (2007) Environment-related life-history trait variations of the red-bellied piranha Pygocentrus nattereri in two river basins of the Bolivian Amazon. J Fish Biol 71:1113–1134
Dutra DL (2010) Estrutura trófica da assembleia de peixes associada a bancos de herbáceas aquáticas em áreas de várzea ao longo do rio Amazonas. Instituto Nacional de Pesquisas da Amazônia. Master dissertation
González R (2006) Nota sobre la presencia del festivo Mesonauta festivus (Heckel, 1840) en el lago Gatún, Panamá. Tecnociencia 8(1):183–189
Goodwin BJ, McAllister AJ, Fahrig L (1999) Predicting invasiveness of plant species based on biological information. Conserv Biol 13:422–426
Gross MR, Sargent RC (1985) The evolution of male and female parental care in fish. Amer Zool 25:807–822
James FC, Johnston RF, Wamer NO, Niemi GJ, Boecklen WJ (1984) The Grinnellian niche of the wood. Am Nat 124(1):17–47
Junk WJ, Bayley PB, Sparks RE (1989) The flood pulse concept in river-floodplain systems. In: Dodge DP (ed) Proceedings of the international large river symposium. Canadian Special Publish Fisheries Aquatic, Science, pp 110–127
Killeen TJ, Schulenberg TS (1998) A biological assessment of Parque Nacional Noel Kempff Mercado, Bolivia. Conservation International, Washington
King M (1995) Fisheries biology assessment and management. Fishing New Books, Massachusetts, 341
Kullander SO (2003) Cichlidae (Cichlids). In: Reis RE, Kullander SO, Ferraris CJJ (eds) Checklist of the freshwater fishes of South and Central America. EDIPUCRS, Porto Alegre, pp 605–654
Kullander SO, Silfvergrip MC (1991) Review of the South American cichlid genus Mesonauta Günther (Teleostei, Cichlidae) with description of two new species. Rev Suisse Zool 98(2):407–448
Lehner PN (1998) Handbook of ethological methods. Cambridge University Press
Lima FCT, Ribeiro A (2011) Continental-scale tectonic controls of biogeography and ecology In: Albert JS and Reis RE (eds.) Historical biogeography of Neotropical freshwater fishes. University of California Press
Lodge DM (1993) Biological invasions: lessons for ecology. Trends Ecol Evol 8:133–137
López-Fernández H, Arbour JH, Winemiller KO, Honeycutt RL (2013) Testing for ancient adaptive radiations in neotropical cichlid fishes. Evol 67(5):1321–1337
Lowe MR, Wu W, Peterson MS, Brown-Peterson NJ, Slack WT, Schofield PJ (2012) Survival, growth and reproduction of non-native Nile tilapia II: fundamental niche projections and invasion potential in the Northern Gulf of Mexico. PLoS ONE 7:e41580
Lowe-McConnell RH (1987) Ecological studies in tropical fish communities. Cambridge University Press, Cambridge, 382
Luiz OJ, Madin JS, Robertson DR, Rocha LA, Wirtz P, Floeter SR (2012) Ecological traits influencing range expansion across large oceanic dispersal barriers: insights from tropical Atlantic reef fishes. Proc R Soc B 279:1033–1040
Lynch M, Gabriel W (1987) Environmental tolerance. Am Nat 129(2):283–303
Malcom JW (2011) Gene networks and metacommunities: dispersal differences can override adaptive advantage. PLoS ONE 6:e21541
McCann K (1998) Density-dependent coexistence in fish communities. Ecology 79:2957–2967
Mérona B, Mol J, Vigouroux R, Chaves PT (2009) Phenotypic plasticity in fish life-history traits in two neotropical reservoirs: Petit-Saut Reservoir in French Guiana and Brokopondo Reservoir in Suriname. Neotrop Ichthyol 7(4):683–692
Moyle PB, Marchetti MP (2006) Predicting invasion success: freshwater fishes in California as a model. Bioscience 56(6):515–524
Olden JD, Poff NL, Bestgen A (2006) Life-history strategies predict fish invasions and extirpations in the Colorado River basin. Ecol Monogr 76(1):25–40
Prado KLL, Freitas CEC, Soares MGM (2010) Assembléias de peixes associadas às macróftas aquáticas em lagos de várzea do baixo rio Solimões. Biotemas 23(1):131–142
Promislow DEL, Montgomerie R, Martin TE (1992) Mortality costs of sexual dimorphism in birds. Proc R Soc Lond B 250:143–150
Promislow DEL, Montgomerie R, Martin TE (1994) Sexual selection and survival in North American waterfowl. Evol 48:2045–2050
Pulliam HR (2000) On the relationship between niche and distribution. Ecol Lett 3(349):361
Röpke CP, Ferreira EF, Zuanon J (2013) Seasonal changes in the use of feeding resources by fish in stands of aquatic macrophytes in an Amazonian floodplain. Braz Environ Biol Fish. doi:10.1007/s10641-013-0160-4
Sanches FHC, Miyai CA, Costa TM, Christofoletti RA, Volpato GL, Barreto RE (2012) Aggressiveness overcomes body-size effects in fights staged between invasive and native fish species with overlapping niches. PLoS ONE 7:e29746
Santos CL, dos Santos IA, da Silva CJ (2009) Ecologia trófica de peixes ocorrentes em bancos de macróftas aquáticas na baia Caiçara, Pantanal Mato-Grossense. Rev Bras Biochem 7(40):473–476
Sargent RC, Taylor PD, Gross MR (1987) Parental care and the evolution of egg size in fishes. Am Nat 129(1):32–46
Sazima I, Carvalho LN, Mendonça FP, Zuanon J (2006) Fallen leaves on the water-bed: diurnal camouflage of three night active fish species in an Amazonian streamlet. Neotrop Ichthyol 4(1):119–122
Schiesari L, Zuanon J, Azevedo-Ramos M, Garcia M, Gordo M, Messias M, Vieira EM (2003) Macrophyte rafts as dispersal vectors for fishes and amphibians in the lower Solimões River, Central Amazon. J Trop Ecol 19:333–336
Schlichting CL (2004) The role of phenotypic plasticity in diversification. In: DeWitt TJ, Scheiner SM (eds) Phenotypic plasticity: Functional and conceptual approach. Oxford University Press, New York, pp 191–200
Schmidt-Nielsen K (1997) Animal physiology: Adaptation and environment. Cambridge University Press, Cambridge, 607
Simpson AC (1951) The fecundity of the plaice. Fish Invest Lond Ser 2(17):27
Sioli H (1951) Zum Alterungsprozess von Flüssen und Flusstypen im Amazonasgebiet. Arch Hydrobiol 45:267–283
Soberón J (2007) Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett 10:1115–1123
Stearns SC (1992) The evolution of life history. Oxford University Press, New York
Veltman CJ, Nee S, Crawley MJ (1996) Correlates of introduction success in exotic New Zealand birds. Am Nat, 542–557
Vila-Gispert A, Alcaraz C, García-Berthou M (2005) Life-history traits of invasive fish in small Mediterranean streams. Biol Invasions 7:107–111
Whittaker RG (1967) Gradient analysis of vegetation. Biol Rev 49:207–264
Winemiller KO (1989) Patterns of variation in life history among South American fishes in seasonal environments. Oecol 81:225–241
Zamprogno C, Andrade GV (1986) Camuflagem em jovens de pacu, Myleus sp. (Characiformes Myleinae). Rev Bras Biol 46(2):415–418
Zaret TM, Paine RT (1973) Species introduction in a tropical lake. Science 182(4111):449–455
Acknowledgments
We are grateful to three anonymous reviewers who provided us with helpful advices. We thank Carolina Doria and Santo Antônio Energia S.A. for allowing the use of LIP-UNIR database, and Lucia Rapp Py-Daniel for kindly providing access to INPA’s fish collection database. We thank the National Council of Scientific and Technological Development (CNPq) for providing scholarship to THSP, JS and CPR and Coordination of Improvement of Higher Education Personnel (CAPES) for providing scholarship to DFC. JZ thanks CNPq for the productivity Grant (#307464/2009-1). The study was conducted according to rules established by INPA’s Ethics Committee (Protocol number: 015/2012). This is the contribution # 34 of the Projeto Igarapés.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Leaf mimicry behavior by a resting Mesonauta festivus adult. (MPG 51208 kb)
Fig. A1
Size distribution of oocytes diameter of each ripe female of Mesonauta festivus (N = 17) from Catalão Lake. BF= Batch fecundity (DOC 486 kb)
Rights and permissions
About this article
Cite this article
Pires, T.H.S., Campos, D.F., Röpke, C.P. et al. Ecology and life-history of Mesonauta festivus: biological traits of a broad ranged and abundant Neotropical cichlid. Environ Biol Fish 98, 789–799 (2015). https://doi.org/10.1007/s10641-014-0314-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-014-0314-z