Abstract
Juvenile Coho Salmon undergo many physiological changes during their springtime transformation from a freshwater parr to a migratory, seawater-capable smolt. Although field observations indicate smolts moving towards the surface and across the breadth of their streams to either swim or drift downstream with the current, water-velocity preferences of these developing cohos are unknown. Using video analysis of their swimming patterns in a calibrated, laboratory flow table with a velocity gradient, groups of three cohos generally increased their preferred water velocity through the springtime study period, to a late-May peak (daytime data, change-point regression analysis, p < 0.05) and over the entire period (nighttime data, regression analysis, p < 0.05). Moving to swifter currents should facilitate the downstream movements of these young cohos, as they develop through the parr-smolt transformation period. This information should assist managers of regulated watersheds and salmon hatcheries in optimizing juvenile salmon survival (e.g., with timely, late-spring water releases producing 0.1–0.3 m s−1 downstream water velocities).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Juvenile Coho Salmon (Oncorhynchus kisutch) undergo many physiological changes during their transformation from a freshwater parr to a migratory, seawater-capable smolt (Loretz et al. 1982; Hoar 1988; Clarke and Hirano 1995). Environmental cues stimulating these physiological changes include photoperiod, temperature, and lunar cycle (Grau et al. 1981, 1982). Coho migrate downstream from mid-March to a peak in mid-May, and through early summer at sub-Alaskan latitudes (reviewed by Sandercock 1991). Field observations from Canada indicate that during the migratory season, coho apparently move towards the surface and across the breadth of their streams, especially during nighttime hours, and either swim or drift downstream with the current (McDonald 1960; Meehan and Siniff 1962). However, no one has quantitatively tested a hypothesis, concerning preferred water velocities of juvenile Coho Salmon during the parr-smolt transformation, under controlled, laboratory conditions.
Recent laboratory-based studies of juvenile Coho Salmon swimming and axial-muscle contractile performance indicate an increased reliance upon passive, rather than active, mechanisms during downstream migration (Katzman and Cech 2001). Such passive mechanisms would be enhanced by these juveniles’ positioning themselves in swifter sections of their natal streams. These seem to fit with the earlier observations of Atlantic Salmon (Salmo salar) smolts’ increased swimbladder gas volumes (reviewed by Wedemeyer et al. 1980) and the decreased, sustained swimming performance in Atlantic Salmon (Thorpe and Morgan 1978) and Coho Salmon smolts (Flagg and Smith 1982), compared with their respective parr life stages. Our objective was to measure the preferred water velocities of juvenile cohos from northern California in a calibrated, laboratory flow table, between April 2 and June 20, 2001. Based on previous field studies (reviewed by Sandercock 1991), we hypothesized that these fish would select faster water velocities as the parr-smolt transformation proceeded during the spring, with a peak in mid-May.
Methods
Experimental fish
Coho Salmon parr (n = 30) were collected from 6°C water at the Iron Gate Hatchery on the Klamath River, California, and transported, in oxygenated, plastic containers, to the Center for Aquatic Biology and Aquaculture (CABA) at the University of California, Davis. They were held, outdoors in 1.2-m diameter, 400-l fiberglass tanks and slowly acclimated (≤1°C·d−1) to 18.5°C. Once at 18.5°C, fish were held for 4 months at CABA, outdoors, under natural (December–March) photoperiods (38°31′ N, 121°30′W), with continuous flows of well water (temperature: 18.5°C, pH: 7.8, hardness: 307 mg·l−1, electrical conductivity: 661 μmhos·cm−1, dissolved O2: 9.0 mg·l−1). Throughout the holding period at CABA, they were fed Rangen® soft-moist salmon feed.
Flow table
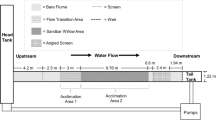
The flow table (208 cm long × 85 cm wide × 18.4 cm deep) used for the experiments was located in a laboratory building incorporating translucent ceiling panels for primary lighting and some fluorescent tubes, for dim, supplemental lighting. The flow table was equipped with three, PVC-pipe water jets on one end and three surface drains at the opposite end to maintain the 12-cm water depth and the 18.4°C (at study’s start) to 18.9°C (at study’s end) water temperature (Fig. 1a). Although the 12-cm water depth seems somewhat shallow, the fish did not respond with any signs of alarm (e.g., rapid, erratic swimming or jumping behaviors). Beecher et al. (2002) found juvenile Coho Salmon occupying many depths in three western-Washington streams, including depths <12 cm. The table’s surface was marked with a 9-cm × 9-cm grid, and an overhead video camera recorded the position of the experimental fish. Each jet was equipped with a ball valve to set the flows, and the velocities (towards the upstream and downstream ends and towards the two sides) were measured at each of the grid lines’ intersections (n = 140) with an electromagnetic flow meter (Marsh-McBirney model 201D, Frederick, Maryland) to calibrate the flow table (Fig. 1b). Negative velocity values resulted from retrograde water movements associated with a large eddy in the slow-current area of the flow table. Spot, water-velocity checks throughout the experiments confirmed the stability of the measured velocity map.
a. Flow table (top view), showing water inlets on right and drains on left. Large arrows generally show the water-velocity gradient, although a large eddy developed in the slower-velocity side of the flow table, with resultant retrograde flows. b. Two-dimensional contour plot of measured velocities (m·s−1) across the flow table. Note the negative velocities in the downstream area of the slower velocity section of the flow gradient (i.e., upper left area on diagram)
Experiments
Three juvenile Coho Salmon were used in each of ten experiments. This fish density approximated that of wild cohos in some streams (Glova 1987). Three segments (early April, late May-early June, and mid-late June) were selected for ten experimental days during the expected parr-smolt transformation period. Between 12:00 and 13:00 hours on each experimental day, three fish were quickly (<30 s) netted from their holding tank and transported, via polyethylene bucket, to the flow table. Fish-position data were recorded, every 10 min, over a 21–23-h period, during daylight hours for all experiments, and during nighttime hours for five, randomly chosen experiments. However, fish-position data for the first hour in the flow table were excluded from the analysis to minimize any stress-related effects of the quick netting and transport to the flow table. Davis and Schreck (1997) found that the oxygen consumption rates of juvenile Coho Salmon returned to pre-stress levels 1 h after the imposition of an acute handling stressor. In the night experiments, the dim, supplemental lighting was left on, allowing videography. The five, nighttime experiments were considered to be a maximum, so as not to re-set the cohos’ biological clocks (see review by Johnsonn and Engelmann 2008) and influence their migratory behavior (see review by Wedemeyer et al. 1980). Videotape data were analyzed by visually locating each of the three fish at the 10-min intervals and calculating their mean velocity preferences for the daytime (all ten experiments) and nighttime (five experiments) hours. Velocity preferences were determined by matching their grid-point locations (i.e., from the grid intersection closest to the fish’s head) and body-axis orientations with velocities from the calibration velocity map. Some of the velocities were “negative,” indicating fish selection of the eddy (retrograde flow) area in the slow-velocity portion of the flow table. Change-point (i.e., piece-wise, Brenden and Bence 2008, daytime data) and least-squares (nighttime data) regressions (SigmaPlot v.11.0, Systat Software Inc., Richmond, California, USA) were used to examine the Coho Salmon’s selected, velocity patterns through the April–June period.
Results
The juvenile Coho Salmon increased in size (from 27.4 g mean wet mass and 143 mm mean TL, to 56.9 g and 179 mm TL) over the study period and gradually appeared more “smolt-like” (loss of parr marks with increased silvering of flanks), as the study progressed.
These juveniles increased their preferred water velocity during the daytime periods from 0.003 m s−1 at the start of the study to a mid-May peak (0.139 m s−1), with a significant, subsequent decline to 0.065 m s−1 (change-point regression, p < 0.05, Fig. 2), supporting our hypothesis. Also, their water velocity preference during nighttime increased, from −0.079 m s−1 to 0.120 m s−1 (least squares regression, p < 0.05, Fig. 2), over the entire study period. Negative velocity preferences were achieved by the fish occupying the eddy section of the flow table, albeit with their heads oriented into the slow, retrograde flow. Overall, daytime mean values were not statistically distinguishable from nighttime mean values, either over the entire study period or during the last month (t-tests, p > 0.05).
Preferred water velocities of juvenile Coho Salmon during day and night through the springtime parr-smolt transformation period. Daytime data showed a significant break-point (piece-wise) regression (p < 0.05) with a peak in mid-May. The regression line represents the linear relationship \( y = 0.0082\,{x_1} + 0.1267-0.0849\left( {{x_1}-53} \right){x_2} \), where x 1 = days from the start of the experiment (2 April 2001), and x 2 = 1 if x 1 > 53, otherwise x 2 = 0 (r 2 = 0.436, n = 30). Nighttime data showed a significant, least-squares regression (p < 0.05), represented by the linear relationship y = 2.766e−3x−0.0858, (r 2 = 0.887, n = 15)
During the earlier experiments (April), the fish mostly stayed in the slower (including eddy) current areas of the flow table. During the later experiments (May and June), they were often swept downstream in the faster currents that they selected. Many of these fish in the later experiments would brace their bodies off of the downstream screen, repeatedly, in the fastest velocity areas (ca. 0.3 m s−1), with their caudal fins (caudal-bracing behavior). For example, after bracing themselves against the screen for several seconds, they would swim in a horizontal looping path through somewhat slower water away from the downstream screen, returning to the swifter currents. The swifter currents would sweep them back to the downstream screen for repeated caudal-bracing and looped-path swimming.
Discussion
Several past studies have shown that smoltification is heavily influenced by photoperiod and that populations of Coho Salmon at sub-Alaskan latitudes in North America undergo the parr-smolt transformation (indicated by increased gill Na+-K+ ATPase activity, sea-water tolerance, body silvering, and downstream migration) during the spring through early summer period (see reviews by Wedemeyer et al. 1980; Sandercock 1991; and Clarke and Hirano 1995). Our data constitute the first measurements of juvenile Coho Salmon’s preferred water velocities in a calibrated, laboratory flow table, during their parr-smolt transformation. Our hypothesis that these fish would select faster water velocities as the parr-smolt transformation proceeded during the spring, with a peak in mid-May, was generally supported, and these laboratory results are in accord with those from previous field studies. Because our fish were exposed to natural photoperiodic and lunar phases during their holding and experimental periods, these environmental factors are probably key (e.g., as opposed to water temperature changes, which were minor in our studies) to their selection of faster water velocities towards the conclusion of this springtime, parr-smolt transformation period.
Coho migrate downstream from mid-March to a peak in mid-May, and through early summer at sub-Alaskan latitudes (reviewed by Sandercock 1991). Although we recorded fewer nighttime data points, both daytime and nighttime data indicated an increasing selection of swifter currents as the parr-smolt transformation progressed (to a peak in mid-May in the daytime data). McMahon and Hartman (1989) described juvenile Coho Salmon behavior in outdoor experimental stream channels; located on Vancouver Island, British Columbia, Canada; which allowed fish to remain in the channels or to emigrate in response to manipulations of cover features and water velocity. These authors observed the pre-emigrating juveniles using velocity refuges, including eddies, induced by simulated root wads and other baffles placed in the stream channels, where velocity was near 0 m s−1. On the other hand, emigrating juveniles moved up in the water column (maximum velocity: 0.14 m s−1), especially near sunset and sunrise, and were swept downstream (McMahon and Hartman 1989). Field observations from Canada indicate that during the migratory season, Coho Salmon apparently move towards the surface and across the breadth of their streams during nighttime hours and either swim or drift downstream with the current (McDonald 1960; Meehan and Siniff 1962). Although our fish were unable to migrate from the flow table, their migratory tendencies in mid-May and June were indicated by their being swept, repeatedly, by the selected, faster currents, to the downstream screen.
The length of juvenile Coho Salmon naturally tends to increase during their pre-emigration, springtime growth and development period in California stream habitats (Shapovalov and Taft 1954). Our fish increased their mean total length by 36 mm over the ca. 12-week period of our experiments. Interestingly, length increases do not result in increased sustained swimming performance in either Atlantic Salmon (Thorpe and Morgan 1978) or Coho Salmon smolts (Flagg and Smith 1982), compared with their respective parr life stages.
Moving to swifter currents should facilitate the downstream movements of migrating, juvenile Coho Salmon, as they develop through the parr-smolt transformation period. Katzman and Cech (2001) found that juvenile Coho Salmon decreased their aerobic swimming performance, measured as critical swimming velocity, when their springtime, parr-smolt transformation was stimulated by intraperitoneal implantation of thyroid-hormone pellets. The same fish showed axial muscle contractility changes ascribed to remodeling of white + red “mosaic” muscle to more of a pure white (fast, glycolytic)-type myotome, compared with sham pellet and control (no implanted pellets) groups (Katzman and Cech 2001). Whereas a decreased population of axial red muscle (slow, oxidative) fibers could explain the decreased critical swimming velocity, the increased population of white fibers would presumably assist in the capture of elusive prey in estuaries, where stream-type drifting prey are unavailable. The faster, more forceful contractions of the juvenile cohos’ axial muscles (Katzman and Cech 2001) should assist evasive, swimming bursts from larger, estuarine predators, also.
Our findings argue that coho smolts are either carried downstream with the river currents or that their active downstream migration is facilitated by these currents, especially towards the end of their smoltification season. Swanson et al. (2004) observed that juvenile Chinook Salmon displayed two swimming patterns when exposed to a “sweeping” current (31 cm s−1) past an annular fish screen, which was situated in an annular flume. Due to their typically shorter residence time in fresh water, Chinook Salmon juveniles migrate at a smaller mean size than do Coho Salmon migrants (reviewed by Healey 1991; Sandercock 1991). The parr-size (4.4–6.4 cm SL) Chinook Salmon at an early-spring stream temperature (12°C) always swam into the current (positive rheotaxis), successfully holding position in the flume (Swanson et al. 2004). In contrast, the smolt-size (6–8 cm SL) fish, at a late-spring temperature (19°C) swam or drifted with the current 69% of the time, averaging ca. 33 cm s−1 as downstream ground speed (ca. 2 cm s−1 faster than the current). Thorpe and Morgan (1978) and Flagg and Smith (1982) provide additional evidence for a strong passive component to downstream migration for Atlantic Salmon and Coho Salmon, respectively.
In much of their North American range, where coastal Coho Salmon populations are endangered in managed watersheds, this new information should assist watershed and hatchery managers in optimizing juvenile salmon survival. For example, Coho Salmon downstream migratory success might be facilitated with timely, late-spring water releases producing 0.1–0.3 m s−1 downstream water velocities.
References
Beecher HA, Caldwell BA, DeMond SB (2002) Evaluation of depth and velocity preferences of juvenile Coho Salmon in Washington streams. North Am J Fish Manage 22:785–795
Brenden TO, Bence JR (2008) Comment: use of piecewise regression models to estimate changing relationships in fisheries. North Am J Fish Manage 28:844–846
Clarke WC, Hirano T (1995) Osmoregulation. In: Groot C, Margolis L, Clarke WC (eds) Physiological ecology of Pacific salmon. UBC, Vancouver, pp 319–377
Davis LE, Schreck CB (1997) The energetic response to handling stress in juvenile Coho Salmon. Trans Am Fish Soc 126:248–258
Flagg TA, Smith LS (1982) Changes in swimming behavior and stamina during smolting of Coho Salmon. In: Brannon EL, Salo EL (eds) Salmon and trout migratory behavior symposium proceedings. University of Washington, Seattle, pp 191–195
Glova GJ (1987) Comparison of allopatric cutthroat trout stocks with those sympatric with Coho Salmon and sculpins in small streams. Environ Biol Fish 20:275–284
Grau EG, Dickoff WW, Nishioka RS, Bern HA, Folmar LC (1981) Lunar phasing of the thyroxine surge preparatory to seaward migration of salmonid fish. Science 211:607–609
Grau EG, Specker JL, Nioshioka RS, Bern HA (1982) Factors determining the surge in thyroid activity in salmon during smoltification. Aquaculture 28:49–57
Healey MC (1991) Life history of Chinook Salmon. In: Groot C, Margolis L (eds) Pacific salmon life histories. UBC, Vancouver, pp 313–393
Hoar WS (1988) The physiology of smolting salmonids. In: Hoar WS, Randall DJ (eds) Fish physiology, vol XIB. Academic, San Diego, pp 275–343
Johnsonn A, Engelmann W (2008) The biological clock and its resetting by light. In: Bjornn LO (ed) Photobiology, 2nd edn. Springer, New York, pp 321–388
Katzman S, Cech JJ Jr (2001) Juvenile Coho Salmon locomotion and mosaic muscle are modified by 3′, 3′, 5′-tri-iodo-L-thyronine. J Exp Biol 204:1711–1717
Loretz CA, Collie NL, Richman NH III, Bern HA (1982) Osmoregulatory changes accompanying smoltification in Coho Salmon. Aquacult 28:67–74
McDonald J (1960) The behaviour of Pacific salmon fry during their downstream migration to freshwater and saltwater nursery areas. J Fish Res Board Can 17:655–676
McMahon TE, Hartman GF (1989) Influence of cover complexity and current velocity on winter habitat use by juvenile Coho Salmon (Oncorhynchus kisutch). Can J Fish Aquat Sci 46:1551–1557
Meehan WR, Siniff DB (1962) A study of the downstream migration of anadromous fishes in the Taku River, Alaska. Trans Am Fish Soc 91:399–467
Sandercock FK (1991) Life history of Coho Salmon. In: Groot C, Margolis L (eds) Pacific salmon life histories. UBC, Vancouver, pp 397–445
Shapovalov L, Taft AC (1954) The life histories of the steelhead rainbow trout (Salmo gairdneri) and silver salmon (Oncorhynchus kisutch) with special emphasis on Waddell Creek, California, and recommendations regarding their management. Calif Dept Fish Game Fish Bull 98:1–375
Swanson C, Young PS, Cech JJ Jr (2004) Swimming in two-vector flows: performance and behavior of juvenile Chinook Salmon near a simulated screened water diversion. Trans Am Fish Soc 133:265–278
Thorpe JE, Morgan RIG (1978) Periodicity in Atlantic Salmon Salmo salar L. smolt migration. J Fish Biol 12:541–548
Wedemeyer GA, Saunders RL, Clarke WC (1980) Environmental factors affecting smoltification and early marine survival of anadromous salmonids. Mar Fish Rev 42(6):1–14
Acknowledgments
We thank K. Rushton and staff of the California Department of Fish and Game’s Iron Gate Hatchery for the Coho Salmon, P. Lutes and E. Hallen (UC Davis Center for Aquatic Biology and Aquaculture) for fish care, H. Nelson and J. Reardon for data analysis assistance, C. Woodley and P. Allen for statistical advice, and V. deVlaming and two anonymous reviewers for helpful comments on the manuscript. Research was supported by fellowships from the Marin Rod and Gun Club, Granite Bay Flycasters, and California Fly Fishers Unlimited, and grants from the UC Davis Jastro-Shields and the Humanities Research Funds (to SMK), and grants from the California Department of Water Resources, U.S. Bureau of Reclamation, CALFED Bay-Delta Program, Anadromous Fish Screen Program (U.S. Bureau of Reclamation and U.S. Fish and Wildlife Service) and the UC Agricultural Experiment Station (Grant No. 3455-H, to JJC).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Katzman, S.M., Greathouse, J., Roessig, J.M. et al. Water velocity preferences of Coho Salmon during the parr-smolt transformation. Environ Biol Fish 88, 79–84 (2010). https://doi.org/10.1007/s10641-010-9619-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-010-9619-8