Summary
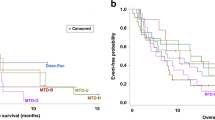
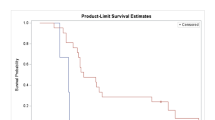
Background The study determined the safety, pharmacokinetics/pharmacodynamics (PK/PD), and recommended Phase II dose of BCT-100 for arginine auxotrophic tumours in a non-Chinese population. Methods This is a Phase I, 3 + 3 dose-escalation, open-label, multi-centre study in two arginine auxotrophic cancers—Malignant Melanoma (MM) and Castration Resistant Prostate Cancer (CRPC). Patients were enrolled to receive weekly intravenous BCT-100. The dose cohorts were respectively 0.5 mg/kg, 1.0 mg/kg, 1.7 mg/kg and 2.7 mg/kg. Results There were 14 MM and 9 CRPC patients, 16 males and 7 females with a median age of 71. No dose-limiting toxicities were reported. Among all the AEs, 18 were drug-related (mostly were Grade 1). Although there were individual variations in PKs amongst the patients in each cohort, the median arginine level was maintained at 2.5 µM (lower limit of quantification) in all 4 cohorts of patients after the second BCT-100 injection. Therapeutic Arginine Depletion was found in the 1.7 and 2.7 mg/kg/week cohorts when anti-tumor activities were observed. The two cohorts had a similar AUC (20,947 and 19,614 h*µg/ml respectively). Since the 2.7 mg/kg/week cohort had a more sustained arginine depletion for 2 weeks, the 2.7 mg/kg/week dose is chosen as the future phase II dose. There were two complete remissions (1 MM & 1 CRPC), 1PR (MM) and 2 stable diseases with a disease control rate (CR + PR + SD) of 5/23 (22%). Conclusions BCT-100 is safe in a non-Chinese population and has anti-tumor activities in both MM and CRPC. Weekly BCT-100 at 2.7 mg/kg is defined as the optimal biological dose for future clinical phase II studies.



Similar content being viewed by others
Availability of data and material
The data set that support the findings of this study are available from the corresponding author on reasonable request.
References
Kishton RJ, Sukumar M, Restifo NP (2016) Arginine Arms T Cells to Thrive and Survive. Cell Metab 24:647–648. https://doi.org/10.1016/j.cmet.2016.10.019
Morris SM (2002) Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr 22:87–105. https://doi.org/10.1146/annurev.nutr.22.110801.140547
Cheng PN-M, Lam T-L, Lam W-M et al (2007) Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res 67:309–317. https://doi.org/10.1158/0008-5472.CAN-06-1945
Scott L, Lamb J, Smith S, Wheatley DN (2000) Single amino acid (arginine) deprivation: rapid and selective death of cultured transformed and malignant cells. Br J Cancer 83:800–810. https://doi.org/10.1054/bjoc.2000.1353
Yau T, Cheng PN, Chan P et al (2015) Preliminary efficacy, safety, pharmacokinetics, pharmacodynamics and quality of life study of pegylated recombinant human arginase 1 in patients with advanced hepatocellular carcinoma. Invest New Drugs 33:496–504. https://doi.org/10.1007/s10637-014-0200-8
Piyarathna DWB, Balasubramanian A, Arnold JM et al (2019) ERR1 and PGC1α associated mitochondrial alterations correlate with pan-cancer disparity in African Americans. J Clin Invest 129:2351–2356. https://doi.org/10.1172/JCI127579
Imaizumi A, Adachi Y, Kawaguchi T et al (2019) Genetic basis for plasma amino acid concentrations based on absolute quantification: a genome-wide association study in the Japanese population. Eur J Hum Genet 27:621–630. https://doi.org/10.1038/s41431-018-0296-y
Schaeffeler E, Eichelbaum M, Brinkmann U et al (2001) Frequency of C3435T polymorphism of MDR1 gene in African people. Lancet 358:383–384. https://doi.org/10.1016/S0140-6736(01)05579-9
Izzo F, Marra P, Beneduce G et al (2004) Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol 22:1815–1822. https://doi.org/10.1200/JCO.2004.11.120
Corbaux P, El-Madani M, Tod M et al (2019) Clinical efficacy of the optimal biological dose in early-phase trials of anti-cancer targeted therapies. Eur J Cancer 120:40–46. https://doi.org/10.1016/j.ejca.2019.08.002
De Santo C, Cheng P, Beggs A et al (2018) Metabolic therapy with PEG-arginase induces a sustained complete remission in immunotherapy-resistant melanoma. J Hematol Oncol 11:68. https://doi.org/10.1186/s13045-018-0612-6
Yau T, Cheng PN, Chan P et al (2013) A phase 1 dose-escalating study of pegylated recombinant human arginase 1 (Peg-rhArg1) in patients with advanced hepatocellular carcinoma. Invest New Drugs 31:99–107. https://doi.org/10.1007/s10637-012-9807-9
Ott PA, Carvajal RD, Pandit-Taskar N et al (2013) Phase I/II study of pegylated arginine deiminase (ADI-PEG 20) in patients with advanced melanoma. Invest New Drugs 31:425–434. https://doi.org/10.1007/s10637-012-9862-2
Kim RH, Coates JM, Bowles TL et al (2009) Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res 69:700–708. https://doi.org/10.1158/0008-5472.CAN-08-3157
Mussai F, Egan S, Hunter S et al (2015) Neuroblastoma Arginase Activity Creates an Immunosuppressive Microenvironment That Impairs Autologous and Engineered Immunity. Cancer Res 75:3043–3053. https://doi.org/10.1158/0008-5472.CAN-14-3443
Mussai F, Egan S, Higginbotham-Jones J et al (2015) Arginine dependence of acute myeloid leukemia blast proliferation: a novel therapeutic target. Blood 125:2386–2396. https://doi.org/10.1182/blood-2014-09-600643
Abou-Alfa GK, Qin S, Ryoo B-Y et al (2018) Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol 29:1402–1408. https://doi.org/10.1093/annonc/mdy101
Yang T-S, Lu S-N, Chao Y et al (2010) A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br J Cancer 103:954–960. https://doi.org/10.1038/sj.bjc.6605856
Diaz GA, Schulze A, McNutt MC et al (2020) Clinical effect and safety profile of pegzilarginase in patients with arginase 1 deficiency. J Inherit Metab Dis. https://doi.org/10.1002/jimd.12343
Chan SL, Cheng PNM, Liu AM et al (2021) A phase II clinical study on the efficacy and predictive biomarker of pegylated recombinant arginase on hepatocellular carcinoma. Invest New Drugs. https://doi.org/10.1007/s10637-021-01111-8
Funding
Bio-Cancer Treatment International Limited.
Author information
Authors and Affiliations
Contributions
PC and AB designed the study and performed research. PC, AL and FM analyzed the data and wrote the manuscript. All authors critically reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by the Institutional Review Boards at all participating centers.
Consent to participate
All patients provided written informed consent prior to enrollment.
Consent for publication
The patient and study members have provided consent for publication.
Conflicts of interest
Dr Paul N.M. Cheng and Dr Angela M. Liu are employees of Bio-Cancer Treatment International Ltd. There are no other conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheng, P.N.M., Liu, A.M., Bessudo, A. et al. Safety, PK/PD and preliminary anti-tumor activities of pegylated recombinant human arginase 1 (BCT-100) in patients with advanced arginine auxotrophic tumors. Invest New Drugs 39, 1633–1640 (2021). https://doi.org/10.1007/s10637-021-01149-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-021-01149-8