Summary
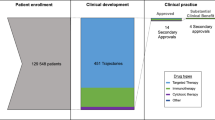
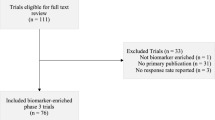
Major advances in cancer care often emerge from the development of novel targets. We randomly sampled 10% of cancer trials on clinicaltrials.gov with start dates 2013–2016 to determine the proportion of trials and research subjects directed at evaluating novel targets. We found that 87 of 378 trials (23.0%) enrolling 9225 of 44,525 patients (20.7%) tested interventions that are directed towards novel targets. 146 of 378 trials (38.6%) enrolling 19,132 of 44,525 patients (43.0%) investigated treatments that were not FDA approved but utilized a previously studied target for treating cancer. Combined, 233 of 378 trials (61.6%) enrolling 28,357 of 44,525 patients (63.9%) investigated treatments that were not FDA approved. Furthermore, 36 of 378 trials (9.5%) enrolling 6592 of 44,525 patients (14.8%) investigated FDA approved anticancer drugs in their approved indication and combination while 109 of 378 trials (28.8%) enrolling 9576 of 44,525 patients (21.5%) investigated FDA approved anticancer drugs outside of their approved indication or combination. Logistic regression found that phase 1 trials were significantly more likely to test novel target interventions than phase 2 and 3 trials (p value = 0.00197 and 0.00130 respectively). Industry sponsored trials were also significantly more likely to involve novel target interventions than non-industry trials (p value <0.001). In conclusion, most cancer trials involve unapproved treatments, but a majority of these treatments are well-characterized or involve a previously studied target to treat cancer.
Similar content being viewed by others
References
Truong TH, Weeks JC, Cook EF, Joffe S (2011) Altruism among participants in cancer clinical trials. Clinical Trials 8(5):616–623. https://doi.org/10.1177/1740774511414444
Carlisle B, Demko N, Freeman G, Hakala A, MacKinnon N, Ramsay T, Hey S, London AJ, Kimmelman J (2016) Benefit, risk, and outcomes in drug development: a systematic review of Sunitinib. J Natl Cancer Inst 108(1). https://doi.org/10.1093/jnci/djv292
Mattina J, Carlisle B, Hachem Y, Fergusson D, Kimmelman J (2017) Inefficiencies and patient burdens in the development of the targeted Cancer drug Sorafenib: a systematic review. PLoS Biol 15(2):e2000487. https://doi.org/10.1371/journal.pbio.2000487
Bertolini F, Sukhatme VP, Bouche G (2015) Drug repurposing in oncology—patient and health systems opportunities. Nat Rev Clin Oncol 12:732–742. https://doi.org/10.1038/nrclinonc.2015.169
Leroux JC (2018) The novelty bubble. J Control Release 278:140–141. https://doi.org/10.1016/j.jconrel.2018.03.032
Naci H, Carter AW, Mossialos E (2015) Why the drug development pipeline is not delivering better medicines. BMJ 351:h5542. https://doi.org/10.1136/bmj.h5542
Administration USFaD (2014) Novel Drugs 2013 Summary
Administration USFaD (2015) Novel Drugs 2014 Summary
Administration USFaD (2016) Novel Drugs 2015 Summary
Administration USFaD (2017) Novel Drugs 2016 Summary
U.S. Food and Drug Administration (2018) Drugs@FDA: FDA Approved Drug Products. https://www.accessdata.fda.gov/scripts/cder/daf/
Hwang TJ, Carpenter D, Lauffenburger JC, Wang B, Franklin JM, Kesselheim AS (2016) Failure of investigational drugs in late-stage clinical development and publication of trial results. JAMA Intern Med 176(12):1826–1833. https://doi.org/10.1001/jamainternmed.2016.6008
Eder J, Sedrani R, Wiesmann C (2014) The discovery of first-in-class drugs: origins and evolution. Nat Rev Drug Discov 13(8):577–587. https://doi.org/10.1038/nrd4336
Gagne JJ, Choudhry NK (2011) How many “me-too” drugs is too many? JAMA 305(7):711–712. https://doi.org/10.1001/jama.2011.152
Acknowledgements
We thank Mark Ratain, Benjamin Carlisle, Deborah Zarin and Patrick Bodilly Kane for advice on this manuscript.
Code availability
There is no custom code or mathematical algorithm that is deemed central to the conclusions of this manuscript.
Funding
This work was supported by the Canadian Institutes of Health Research in Ottawa, Canada. CIHR (EOG 111391).
Manuscript Writing: EG, JK.
Author information
Authors and Affiliations
Contributions
Trial Extraction and Screening: AF,SW.
Novelty Analysis: RB, EG, NH.
Corresponding author
Ethics declarations
Conflict of interest
Eli Gumnit declares that he has no conflict of interest. Aden C. Feustel declares that he has no conflict of interest. Sandy Wong declares that she has no conflict of interest. Rafia Bosan declares that she has no conflict of interest. Nora Hutchinson declares that she has no conflict of interest. Jonathan Kimmelman serves in a remunerative capacity on a DSMB for Ultragenyx Inc.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. All information was gathered from publicly available databases.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gumnit, E., Feustel, A.C., Wong, S. et al. The proportion of North American cancer trials that evaluate novel targets. Invest New Drugs 39, 256–259 (2021). https://doi.org/10.1007/s10637-020-00971-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00971-w