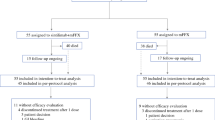
Summary
Background Ruxolitinib, a Janus kinase 1 (JAK1)/JAK2 inhibitor, plus capecitabine improved overall survival (OS) vs capecitabine in a subgroup analysis of patients with metastatic pancreatic cancer and systemic inflammation (C-reactive protein [CRP] >13 mg/dL) in the randomized phase II RECAP study. We report results from two randomized phase III studies, JANUS 1 (NCT02117479) and JANUS 2 (NCT02119663). Patients and Methods Adults with advanced/metastatic pancreatic cancer, one prior chemotherapy regimen and CRP >10 mg/L were randomized 1:1 (stratified by modified Glasgow Prognostic Score [1 vs 2] and Eastern Cooperative Oncology Group performance status [0/1 vs 2]) to 21-day cycles of ruxolitinib 15 mg twice daily plus capecitabine 2000 mg/m2/day (Days 1–14) or placebo plus capecitabine. The primary endpoint was OS. Results Both studies were terminated following a planned interim futility/efficacy analysis of JANUS 1. Overall, 321 and 86 patients were randomized in JANUS 1 (ruxolitinib: n = 161; placebo: n = 160) and JANUS 2 (ruxolitinib: n = 43; placebo: n = 43). There was no significant difference in OS or progression-free survival (PFS) between treatments in JANUS 1 (OS: hazard ratio [HR], 0.969, 95% confidence interval [CI], 0.747–1.256; PFS: HR, 1.056; 95% CI, 0.827–1.348) or JANUS 2 (OS: HR, 1.584; 95% CI, 0.886–2.830; PFS: HR, 1.166; 95% CI, 0.687–1.978). The most common hematologic adverse event was anemia. No new safety signals with ruxolitinib or capecitabine were identified. Conclusions Ruxolitinib plus capecitabine was well tolerated in refractory pancreatic cancer patients; this combination did not improve survival.



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30. https://doi.org/10.3322/caac.21387
NCCN clinical practice guidelines in oncology: Pancreatic adenocarcinoma. Version 2.2017. National Comprehensive Cancer Network website http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed 16 August 2017
Walker EJ, Ko AH (2014) Beyond first-line chemotherapy for advanced pancreatic cancer: an expanding array of therapeutic options? World J Gastroenterol 20:2224–2236. https://doi.org/10.3748/wjg.v20.i9.2224
Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, Hubner RA, Chiu CF, Schwartsmann G, Siveke JT, Braiteh F, Moyo V, Belanger B, Dhindsa N, Bayever E, Von Hoff DD, Chen LT (2016) Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 387:545–557. https://doi.org/10.1016/S0140-6736(15)00986-1
ONIVYDE® (irinotecan liposome injection) [package insert] (2015) Cambridge, Merrimack Pharmaceuticals, Inc
Quintás-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle PA, Caulder E, Wen X, Li Y, Waeltz P, Rupar M, Burn T, Lo Y, Kelley J, Covington M, Shepard S, Rodgers JD, Haley P, Kantarjian H, Fridman JS, Verstovsek S (2010) Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood 115:3109–3117. https://doi.org/10.1182/blood-2009-04-214957
JAKAVI® (ruxolitinib) [summary of product characteristics] (2016) Camberley, UK, Novartis Europharm Limited. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002464/WC500133223.pdf. Accessed 8 May 2017
JAKAFI® (ruxolitinib) [package insert] (2016) Wilmington, DE, Incyte Corporation. http://www.jakafi.com/pdf/prescribing-information.pdf. Accessed 8 May 2017
Gore J, Craven KE, Wilson JL, Cote GA, Cheng M, Nguyen HV, Cramer HM, Sherman S, Korc M (2015) TCGA data and patient-derived orthotopic xenografts highlight pancreatic cancer-associated angiogenesis. Oncotarget 6:7504–7521. https://doi.org/10.18632/oncotarget.3233
Koblish HK, Hansbury M, Wang L-CS, Yang G, Huang T, Xue C-B, Li Y-L, Yue E, Combs A, Yao W, Huber R, Scherle P (2015) Novel immunotherapeutic activity of JAK and PI3Kδ inhibitors in a model of pancreatic cancer [abstract]. Cancer Res 75:1336. https://doi.org/10.1158/1538-7445.am2015-1336
Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Klöppel G, Yoshimura A, Reindl W, Sipos B, Akira S, Schmid RM, Algül H (2011) Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 19:456–469. https://doi.org/10.1016/j.ccr.2011.03.009
Tan CR, Yaffee PM, Jamil LH, Lo SK, Nissen N, Pandol SJ, Tuli R, Hendifar AE (2014) Pancreatic cancer cachexia: a review of mechanisms and therapeutics. Front Physiol 5(88). https://doi.org/10.3389/fphys.2014.00088
Allin KH, Nordestgaard BG (2011) Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci 48:155–170. https://doi.org/10.3109/10408363.2011.599831
Salmiheimo A, Mustonen H, Stenman U-H, Puolakkainen P, Kemppainen E, Seppänen H, Haglund C (2016) Systemic inflammatory response and elevated tumour markers predict worse survival in resectable pancreatic ductal adenocarcinoma. PLoS One 11:e0163064. https://doi.org/10.1371/journal.pone.0163064
McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS (2007) Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Color Dis 22:881–886. https://doi.org/10.1007/s00384-006-0259-6
McMillan DC (2013) The systemic inflammation-based Glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treat Rev 39:534–540. https://doi.org/10.1016/j.ctrv.2012.08.003
Hurwitz HI, Uppal N, Wagner SA, Bendell JC, Beck JT, Wade SM 3rd, Nemunaitis JJ, Stella PJ, Pipas JM, Wainberg ZA, Manges R, Garrett WM, Hunter DS, Clark J, Leopold L, Sandor V, Levy RS (2015) Randomized, double-blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J Clin Oncol 33:4039–4047. https://doi.org/10.1200/JCO.2015.61.4578
Heffernan N, Cella D, Webster K, Odom L, Martone M, Passik S, Bookbinder M, Fong Y, Jarnagin W, Blumgart L (2002) Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. J Clin Oncol 20:2229–2239. https://doi.org/10.1200/JCO.2002.07.093
Sonbol MB, Firwana B, Wang Z, Almader-Douglas D, Borad MJ, Makhoul I, Ramanathan RK, Ahn DH, Bekaii-Saab T (2017) Second-line treatment in patients with pancreatic ductal adenocarcinoma: a meta-analysis. Cancer 123:4680–4686. https://doi.org/10.1002/cncr.30927
Cinar P, Ko AH (2017) Best practices for the treatment of metastatic pancreatic adenocarcinoma: the therapeutic landscape in 2017. Chin Clin Oncol 6:29. https://doi.org/10.21037/cco.2017.06.13
Aprile G, Negri FV, Giuliani F, De Carlo E, Melisi D, Simionato F, Silvestris N, Brunetti O, Leone F, Marino D, Santini D, Dell'Aquila E, Zeppola T, Puzzoni M, Scartozzi M (2017) Second-line chemotherapy for advanced pancreatic cancer: which is the best option? Crit Rev Oncol Hematol 115:1–12. https://doi.org/10.1016/j.critrevonc.2017.03.025
Imrie CW (2015) Host systemic inflammatory response influences outcome in pancreatic cancer. Pancreatology 15:327–330. https://doi.org/10.1016/j.pan.2015.04.004
Jamieson NB, Denley SM, Logue J, MacKenzie DJ, Foulis AK, Dickson EJ, Imrie CW, Carter R, McKay CJ, McMillan DC (2011) A prospective comparison of the prognostic value of tumor- and patient-related factors in patients undergoing potentially curative surgery for pancreatic ductal adenocarcinoma. Ann Surg Oncol 18:2318–2328. https://doi.org/10.1245/s10434-011-1560-3
Muranaka T, Kuwatani M, Komatsu Y, Sawada K, Nakatsumi H, Kawamoto Y, Yuki S, Kubota Y, Kubo K, Kawahata S, Kawakubo K, Kawakami H, Sakamoto N (2017) Comparison of efficacy and toxicity of FOLFIRINOX and gemcitabine with nab-paclitaxel in unresectable pancreatic cancer. J Gastrointest Oncol 8:566–571. https://doi.org/10.21037/jgo.2017.02.02
Ostojic A, Vrhovac R, Verstovsek S (2012) Ruxolitinib for the treatment of myelofibrosis: its clinical potential. Ther Clin Risk Manag 8:95–103. https://doi.org/10.2147/TCRM.S23277
Wörmann SM, Song L, Ai JY, Diakopoulos KN, Kurkowski MU, Görgülü K, Ruess D, Campbell A, Doglioni C, Jodrell D, Neesse A, Demir IE, Karpathaki AP, Barenboim M, Hagemann T, Rose-John S, Sansom O, Schmid RM, Protti MP, Lesina M, Algül H (2016) Loss of P53 function activates JAK2-STAT3 signaling to promote pancreatic tumor growth, stroma modification, and gemcitabine resistance in mice and is associated with patient survival. Gastroenterology 151:180–193. https://doi.org/10.1053/j.gastro.2016.03.010
Smigiel JM, Parameswaran N, Jackson MW (2017) Potent EMT and CSC phenotypes are induced by oncostatin-M in pancreatic cancer. Mol Cancer Res 15:478–488. https://doi.org/10.1158/1541-7786.mcr-16-0337
Mascarenhas J, Hoffman R (2012) Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res 18:3008–3014. https://doi.org/10.1158/1078-0432.CCR-11-3145
Kremyanskaya M, Atallah EL, Hoffman R, Mascarenhas JO (2013) Clarifying the use of ruxolitinib in patients with myelofibrosis. Oncology 27:706–714
Mascarenhas J, Mughal TI, Verstovsek S (2012) Biology and clinical management of myeloproliferative neoplasms and development of the JAK inhibitor ruxolitinib. Curr Med Chem 19:4399–4413
von Manstein V, Yang CM, Richter D, Delis N, Vafaizadeh V, Groner B (2013) Resistance of cancer cells to targeted therapies through the activation of compensating signaling loops. Curr Signal Transduct Ther 8:193–202. https://doi.org/10.2174/1574362409666140206221931
Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A (2016) Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 375:819–829. https://doi.org/10.1056/NEJMoa1604958
Yu H, Kortylewski M, Pardoll D (2007) Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 7:41–51. https://doi.org/10.1038/nri1995
Brookmeyer R, Crowley JJ (1982) A confidence interval for the median survival time. Biometrics 38:29–41
Acknowledgments
The authors wish to thank the patients and their families, the investigators, and the site personnel who participated in this study. This study was sponsored by Incyte Corporation (Wilmington, DE, USA). Medical writing assistance was provided by Sneha D’Silva, MD, on behalf of Evidence Scientific Solutions Inc. (Philadelphia, PA, USA), and funded by Incyte.
Funding
This study was sponsored by Incyte Corporation (Wilmington, DE, USA).
Author information
Authors and Affiliations
Contributions
Conception/design of the work: Hurwitz, Van Cutsem, Bendell, Hidalgo, Li, Garrido, Macarulla, Sahai, Sama, Greeno, Yu, Verslype, Clark, O’Reilly.
Acquisition of data: Hurwitz, Van Cutsem, Bendell, Hidalgo, Li, Garrido, Macarulla, Sahai, Sama, Greeno, Yu, Verslype, O’Reilly.
Data analysis and interpretation: Dawkins, Walker, Clark.
Writing, review, and revision of the manuscript: All authors.
Final approval of the manuscript: All authors.
Corresponding author
Ethics declarations
Conflicts of interest
Hurwitz: Honoraria – Genentech/Roche, Lilly/ImClone; Consulting or advisory role – Acceleron Pharma, Bristol-Myers Squibb, Genentech/Roche, GlaxoSmithKline, Incyte, Lilly, Novartis, OncoMed, TRACON Pharma; Research funding – Bristol-Myers Squibb (Inst), Genentech/Roche (Inst), GlaxoSmithKline (Inst), Lilly (Inst), Macrogenics (Inst), NCI (Inst), Novartis (Inst), Regeneron (Inst), TRACON Pharma (Inst); Employment – Genentech/Roche as of 23 October 2017. Van Cutsem: Research funding – Amgen (Inst), Bayer (Inst), Boehringer Ingelheim (Inst), Celgene (Inst), Ipsen (Inst), Lilly (Inst), Merck Serono (Inst), Novartis (Inst), Roche (Inst), Sanofi (Inst). Bendell: Research funding – Incyte. Sahai: Consulting – Celgene, Halozyme, NewLink; Research funding – Agios (Inst), Bristol-Myers Squibb (Inst), Celgene (Inst), Incyte (Inst). Sama: Honoraria – OncoPlex Diagnostics; Research funding – Bristol-Myers Squibb (Inst), Incyte (Inst), MedImmune (Inst), OncoMed (Inst), Precision Biologics (Inst); Employment – Bristol-Myers Squibb (Inst) as of March 20, 2017. Yu: Consulting or advisory role – Celgene, Merck Serono, Merrimack. Dawkins, Walker, Clark: Employment – Incyte; Stock and other ownership interests – Incyte. O’Reilly: Consulting or advisory role – Aduro Biotech, Astellas Pharma (Inst), Celgene, Celsion (Inst), Cipla, Exelixis (Inst), Gilead Sciences, Hexal (Inst), IntegraGen (Inst), Jennerex (Inst), Lilly (Inst), MedImmune, Merrimack, Novartis (Inst), Pharmacyclics, Sanofi, Silenseed, Vicus Therapeutics; Research funding – AstraZeneca/MedImmune (Inst), Bristol-Myers Squibb (Inst), Celgene (Inst), Immunomedics (Inst), Incyte (Inst), Momenta Pharmaceuticals (Inst), Myriad Genetics (Inst), OncoMed (Inst), Polaris (Inst), Sanofi (Inst). Hidalgo, Li, Garrido, Macarulla, Greeno, Verslype: Nothing to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hurwitz, H., Van Cutsem, E., Bendell, J. et al. Ruxolitinib + capecitabine in advanced/metastatic pancreatic cancer after disease progression/intolerance to first-line therapy: JANUS 1 and 2 randomized phase III studies. Invest New Drugs 36, 683–695 (2018). https://doi.org/10.1007/s10637-018-0580-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0580-2