Summary
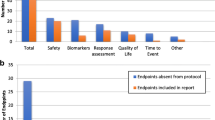
Background Data on completeness of reporting of phase I cancer clinical trials in publications are lacking. Methods The ClinicalTrials.gov database was searched for completed adult phase I cancer trials with reported results. PubMed was searched for matching primary publications published prior to November 1, 2016. Reporting in primary publications was compared with the ClinicalTrials.gov database using a 28-point score (2=complete; 1=partial; 0=no reporting) for 14 items related to study design, outcome measures and safety profile. Inconsistencies between primary publications and ClinicalTrials.gov were recorded. Linear regression was used to identify factors associated with incomplete reporting. Results After a review of 583 trials in ClinicalTrials.gov, 163 matching primary publications were identified. Publications reported outcomes that did not appear in ClinicalTrials.gov in 25% of trials. Outcomes were upgraded, downgraded or omitted in publications in 47% of trials. The overall median reporting score was 23/28 (interquartile range 21–25). Incompletely reported items in >25% publications were: inclusion criteria (29%), primary outcome definition (26%), secondary outcome definitions (53%), adverse events (71%), serious adverse events (80%) and dates of study start and database lock (91%). Higher reporting scores were associated with phase I (vs phase I/II) trials (p<0.001), multicenter trials (p<0.001) and publication in journals with lower impact factor (p=0.004). Conclusions Reported results in primary publications for early phase cancer trials are frequently inconsistent or incomplete compared with ClinicalTrials.gov entries. ClinicalTrials.gov may provide more comprehensive data from new cancer drug trials.


Similar content being viewed by others
References
Di Maio M, Gallo C, Leighl NB et al (2015) Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol 33(8):910–915
Fromme EK, Eilers KM, Mori M et al (2004) How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the quality-of-life questionnaire C30. J Clin Oncol 22(17):3485–3490
Basch E, Iasonos A, McDonough T et al (2006) Patient versus clinician symptom reporting using the National Cancer Institute common terminology criteria for adverse events: results of a questionnaire- based study. Lancet Oncol 7(11):903–909
Cirillo M, Venturini M, Ciccarelli L et al (2009) Clinician versus nurse symptom reporting using the National Cancer Institute-common terminology criteria for adverse events during chemotherapy: results of a comparison based on patient’s self-reported questionnaire. Ann Oncol 20(12):1929–1935
Sivendran S, Latif A, McBride RB et al (2014) Adverse event reporting in cancer clinical trial publications. J Clin Oncol 32(2):83–89
Riveros C, Dechartres A, Perrodeau E et al (2013) Timing and completeness of trial results posted at ClinicalTrials.gov and published in journals. PLoS Med 10(12):e1001566 discussion e1001566
Vera-Badillo FE, Napoleone M, Krzyzanowska MK et al (2016) Bias in reporting of randomised clinical trials in oncology. Eur J Cancer 61:29–35
Tannock IF, Amir E, Booth CM et al (2016) Relevance of randomised controlled trials in oncology. Lancet Oncol 17(12):e560–e567
Boutron I, Altman DG, Hopewell S et al (2014) Impact of spin in the abstracts of articles reporting results of randomized controlled trials in the field of cancer: the SPIIN randomized controlled trial. J Clin Oncol 32(36):4120–4126
Camacho LH, Bacik J, Cheung A, Spriggs DR (2005) Presentation and subsequent publication rates of phase I oncology clinical trials. Cancer 104(7):1497–1504
ClinicalTrials.gov. About the Results Database. https://clinicaltrials.gov/ct2/about-site/results. Last accessed June 19, 2017
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 22(3):276–282
Gerber DE, Pruitt SL, Halm EA (2015) Should criteria for inclusion in cancer clinical trials be expanded? J Comp Eff Res 4(4):289–291
Lemieux J, Goodwin PJ, Pritchard KI et al (2008) Identification of cancer care and protocol characteristics associated with recruitment in breast cancer clinical trials. J Clin Oncol 269270:4458–4465
Srikanthan A, Vera-Badillo F, Ethier J et al (2016) Evolution in the eligibility criteria of randomized controlled trials for systemic cancer therapies. Cancer Treat Rev 43:67–73
Blumle A, Meerpohl JJ, Rucker G et al (2011) Reporting of eligibility criteria of randomized trials: cohort study comparing trial protocols with subsequent articles. BMJ 342:d1828
Kim ES, Bernstein D, Hilsenbeck SG et al (2015) Modernizing eligibility criteria for molecularly driven trials. J Clin Oncol 33(25):2815–2820
Van Spall HG, Toren A, Kiss A, Fowler RA (2007) Eligibility criteria of randomized controlled trials published in high-impact general medical. JAMA 297(11):1233–1240
Begg CB, Engstrom PF (1987) Eligibility and extrapolation in cancer clinical trials. J Clin Oncol 5(6):962–968
Zhang S, Liang F, Li W, Tannock I (2016) Comparison of eligibility criteria between protocols, registries, and publications of cancer clinical trials. J Natl Cancer Inst 108(11):djw129
Mathieu S, Boutron I, Moher D et al (2009) Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 302(9):977–984
Dwan K, Gamble C, Williamson PR, Kirkham JJ, Reporting Bias Group (2013) Systematic review of the empirical evidence of study publication bias and outcome reporting bias - an updated review. PLoS One 8(7):e66844
Le Tourneau C, Razak AR, Gan HK et al (2011) Heterogeneity in the definition of dose-limiting toxicity in phase I cancer clinical trials of molecularly targeted agents: a review of the literature. Eur J Cancer 47(10):1468–1475
Dal-Ré R, Marušić A (2016) Prevention of selective outcome reporting: let us start from the beginning. Eur J Clin Pharmacol 72(10):1283–1288
Mills JL (1993) Data torturing. N Engl J Med 329(16):1196–1199
Dechartres A, Ravaud P, Atal I et al (2016) Association between trial registration and treatment effect estimates: a meta-epidemiological study. BMC Med 14(1):100
Ioannidis JP, Evans SJ, Gøtzsche PC et al (2004) Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med 141(10):781–788
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors have declared no conflicts of interests
Rights and permissions
About this article
Cite this article
Shepshelovich, D., Goldvaser, H., Wang, L. et al. Comparison of reporting phase I trial results in ClinicalTrials.gov and matched publications. Invest New Drugs 35, 827–833 (2017). https://doi.org/10.1007/s10637-017-0510-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0510-8