Summary
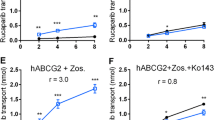
BMS-275,183 is a novel oral C-4 methyl carbonate analogue of paclitaxel. Recently, a drug-drug interaction between BMS-275,183 and benzimidazole proton pump inhibitors (PPIs) was suggested in clinical trials resulting in elevated drug exposure and toxicity. We explored whether the interaction takes place at the level of P-glycoprotein (Pgp, MDR1, ABCB1), Breast Cancer Resistance Protein (BCRP, ABCG2) and MRP2 (ABCC2) using in vitro and in vivo models. In vitro cell survival, drug accumulation, efflux and transport studies with BMS-275,183 were performed employing MDCKII (wild-type, MDR1, BCRP, MRP2) and LLCPK (wild-type and MDR1) cells. In vivo the pharmacokinetics and tissue distribution of BMS-275,183 after p.o. and i.v. administration were explored in Mdr1a/1b−/− and wild-type mice, in presence or absence of the PPI pantoprazole. Results In vitro, BMS-275,183 was found to be a good substrate for MDR1, a moderate substrate for MRP2 and not a substrate for BCRP. In vivo, oral bioavailability, plasma AUC0-6h and brain concentrations were significantly 1.5–, 4–, and 2-fold increased, respectively, in Mdr1a/1b−/− compared with wild-type mice (p < 0.001). However, oral co-administration of pantoprazole (40 mg/kg) did not alter the pharmacokinetics of BMS-275,183 in wild-type mice. Conclusions BMS-275,183 is efficiently transported by Pgp and to a lesser extent by MRP2 in vitro. Genetic deletion of Pgp significantly altered the pharmacokinetics and brain distribution of p.o. and i.v. administered BMS-275,183 in Mdr1a/1b−/− compared to wild-type mice. Oral co-administration of BMS-275,183 with pantoprazole did not affect the pharmacokinetics of BMS-275,183 in wild-type mice, suggesting no interaction with PPI at the dose employed.





Similar content being viewed by others
References
Mastalerz H, Cook D, Fairchild CR, Hansel S, Johnson W, Kadow JF, Long BH, Rose WC, Tarrant J, Wu MJ, Xue MQ, Zhang G, Zoeckler M, Vyas DM (2003) The discovery of BMS-275183: an orally efficacious novel taxane. Bioorg Med Chem 11:4315–4323
Sparreboom A, van Asperen J, Mayer U, Schinkel AH, Smit JW, Meijer DK, Borst P, Nooijen WJ, Beijnen JH, van Tellingen O (1997) Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc Natl Acad Sci U S A 94:2031–2035
Huizing MT, Giaccone G, van Warmerdam LJ, Rosing H, Bakker PJ, Vermorken JB, Postmus PE, van Zandwijk N, Koolen MG, ten Bokkel Huinink WW, van der Vijgh WJ, Bierhorst FJ, Lai A, Dalesio O, Pinedo HM, Veenhof CH, Beijnen JH (1997) Pharmacokinetics of paclitaxel and carboplatin in a dose-escalating and dose-sequencing study in patients with non-small-cell lung cancer. J Clin Oncol 15:317–329
Rose WC, Long BH, Fairchild CR, Lee FY, Kadow JF (2001) Preclinical pharmacology of BMS-275183, an orally active taxane. Clin Cancer Res 7:2016–2021
Liu G, Franssen E, Fitch MI, Warner E (1997) Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol 15:110–115
Bröker LE, de Vos FY, van Groeningen CJ, Kuenen BC, Gall HE, Woo MH, Voi M, Gietema JA, de Vries EG, Giaccone G (2006) Phase I trial with BMS-275183, a novel oral taxane with promising antitumor activity. Clin Cancer Res 12:1760–1767
Bröker LE, Veltkamp SA, Heath EI, Kuenen BC, Gall H, Astier L, Parker S, Kayitalire L, Lorusso PM, Schellens JH, Giaccone G (2007) A phase I safety and pharmacologic study of a twice weekly dosing regimen of the oral taxane BMS-275183. Clin Cancer Res 13:3906–3912
Heath EI, Lorusso P, Ramalingam SS, Awada A, Egorin MJ, Besse-Hamer T, Cardoso F, Valdivieso M, Has T, Alland L, Zhou X, Belani CP (2011) A phase 1 study of BMS-275183, a novel oral analogue of paclitaxel given on a daily schedule to patients with advanced malignancies. Invest New Drugs 29:1426–1431
Schiller JH, Storer B, Tutsch K, Arzoomanian R, Alberti D, Feierabend C, Spriggs D (1994) Phase I trial of 3-h infusion of paclitaxel with or without granulocyte colony-stimulating factor in patients with advanced cancer. J Clin Oncol 12:241–248
Ly VT, Caceres-Cortes J, Zhang D, Humphreys WG, Ekhato IV, Everett D, Cömezoğlu SN (2009) Metabolism and excretion of an oral taxane analog, [14C] 3′-tert-butyl-3′-N-tert-butyloxycarbonyl-4-deacetyl-3′-dephenyl-3′-N-debenzoyl-4-O-methoxy-paclitaxel (BMS-275183), in rats and dogs. Drug Metab Dispos 37:1115–1128
Zhang D, Ly VT, Lago M, Tian Y, Gan J, Humphreys WG, Cömezoglu SN (2009) CYP3A4-mediated ester cleavage as the major metabolic pathway of the oral taxane 3′-tert-butyl-3′-N-tert-butyloxycarbonyl-4-deacetyl-3′-dephenyl-3′-N-debenzoyl-4-O-methoxycarbonyl-paclitaxel (BMS-275183). Drug Metab Dispos 37:710–718
Pauli-Magnus C, Rekersbrink S, Klotz U, Fromm MF (2001) Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn Schmiedeberg’s Arch Pharmacol 364:551–557
Breedveld P, Zelcer N, Pluim D, Sönmezer O, Tibben MM, Beijnen JH, Schinkel AH, van Tellingen O, Borst P, Schellens JH (2004) Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions. Cancer Res 64:5804–5811
Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, Schellens JH (2005) The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res 65:2577–2582
Marchetti S, Mazzanti R, Beijnen JH, Schellens JHM (2007) Clinical relevance: drug-drug interaction, pharmacokinetics, pharmacodynamics, and toxicity. Drug Transporters, Molecular Characterization and role in Drug Disposition. John Wiley & Sons. 24:747–880
Horio M, Chin KV, Currier SJ, Goldenberg S, Williams C, Pastan I, Gottesman MM, Handler J (1989) Transepithelial transport of drugs by the multidrug transporter in cultured Madin-Darby canine kidney cell epithelia. J Biol Chem 264:14880–14884
Evers R, Kool M, van Deemter L, Janssen H, Calafat J, Oomen LC, Paulusma CC, Oude Elferink RP, Baas F, Schinkel AH, Borst P (1998) Drug export activity of the human canalicular multispecific organic anion transporter in polarized kidney MDCK cells expressing cMOAT (MRP2) cDNA. J Clin Invest 101:1310–1319
Pavek P, Merino G, Wagenaar E, Bolscher E, Novotna M, Jonker JW, Schinkel AH (2005) Human breast cancer resistance protein: interactions with steroid drugs, hormones, the dietary carcinogen 2-amino-1-methyl-6-phenylimidazol (4,5-b-)-pyridine, and transport of Cimetidine. J Pharmacol Exp Ther 312:144–152
Mistry P, Kelland LR, Abel G, Sidhal S, Harrap KR (1991) The relationships between glutathione, glutathione-S-transferase and cytotoxicity of platinum drugs and melphalan in eight ovarian carcinoma cell lines. Br J Cancer 64:215–220
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:48–54
Schinkel AH, Mayer U, Wagenaar E, Mol CA, van Deemter L, Smit JJ, van der Valk MA, Voordouw AC, Spits H, van Tellingen O, Zijlmans JM, Fibbe WE, Borst P (1997) Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci U S A 94:4028–4033
Sparreboom A, van Tellingen O, Nooijen WJ, Beijnen JH (1995) Determination of paclitaxel and metabolites in mouse plasma, tissues, urine and faeces by semi-automated reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Appl 664:383–391
Tang R, Faussat AM, Perrot JY, Marjanovic Z, Cohen S, Storme T, Morjani H, Legrand O, Marie JP (2008) Zosuquidar restores drug sensitivity in P-glycoprotein expressing acute myeloid leukemia (AML). BMC Cancer 8:51. doi:10.1186/1471-2407-8-51
Huisman MT, Chhatta AA, van Tellingen O, Beijnen JH, Schinkel AH (2005) MRP2 (ABCC2) transports taxanes and confers paclitaxel resistance and both processes are stimulated by probenecid. Int J Cancer 116:824–829
Lagas JS, Vlaming ML, van Tellingen O, Wagenaar E, Jansen RS, Rosing H, Beijnen JH, Schinkel AH (2006) Multidrug resistance protein 2 is an important determinant of paclitaxel pharmacokinetics. Clin Cancer Res 12:6125–6132
Hendrikx JJ, Lagas JS, Rosing H, Schellens JH, Beijnen JH, Schinkel AH (2013) P-glycoprotein and cytochrome P450 3A act together in restricting the oral bioavailability of paclitaxel. Int J Cancer 132:2439–2447
CPMP/EWP/560/95/Rev.1 Guideline on the Investigation of Drug Interactions. June 2012. www.ema.eu
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for Industry Drug Interaction Studies — Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. February 2012. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm
International Transporter Consortium, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L (2010) Membrane transporters in drug development. Nat Rev Drug Discov 9:215–236
Giacomini KM, Huang SM (2013) Transporters in drug development and clinical pharmacology. Clin Pharmacol Ther 94:3–9. doi:10.1038/clpt.2013.86
van Waterschoot RA, Lagas JS, Wagenaar E, van der Kruijssen CM, van Herwaarden AE, Song JY, Rooswinkel RW, van Tellingen O, Rosing H, Beijnen JH, Schinkel AH (2009) Absence of both cytochrome P450 3A and P-glycoprotein dramatically increases docetaxel oral bioavailability and risk of intestinal toxicity. Cancer Res 69:8996–9002
Smith NF, Acharya MR, Desai N, Figg WD, Sparreboom A (2005) Identification of OATP1B3 as a high-affinity hepatocellular transporter of paclitaxel. Cancer Biol Ther 4:815–818
van de Steeg E, van Esch A, Wagenaar E, van der Kruijssen CM, van Tellingen O, Kenworthy KE, Schinkel AH (2011) High impact of Oatp1a/1b transporters on in vivo disposition of the hydrophobic anticancer drug paclitaxel. Clin Cancer Res 17:294–301. doi:10.1158/1078-0432.CCR-10-1980
Bröker LE, Valdivieso M, Pilat MJ, Deluca P, Zhou X, Parker S, Giaccone G, Lorusso PM (2008) Effect of food on the pharmacokinetic behavior of the potent oral taxane BMS-275183. Clin Cancer Res 14:4186–4191
Jabir RS, Naidu R, Annuar MA, Ho GF, Munisamy M, Stanslas J (2006) Pharmacogenetics of taxanes: impact of gene polymorphisms of drug transporters on pharmacokinetics and toxicity. Pharmacogenomics 13:1979–1988
Lévy P, Gligorov J, Antoine M, Rezai K, Lévy E, Selle F, Saintigny P, Lokiec F, Avenin D, Beerblock K, Lotz JP, Bernaudin JF, Fajac A (2013) Influence of ABCB1 polymorphisms and docetaxel pharmacokinetics on pathological response to neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat 139:421–428. doi:10.1007/s10549-013-2545-7
Acknowledgments
We thank M.A.J. van Eijndhoven and M.J. Boljin (Departments of Molecular Pathology and Clinical Pharmacology, The Netherlands Cancer Institute) for the precious technical assistance.
Disclosures
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Serena Marchetti and Dick Pluim equally contributed to the publication
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure 6
(Supplementary figure for the website). Chemical structure of BMS-275,183, paclitaxel and docetaxel (DOC 71 kb)
Rights and permissions
About this article
Cite this article
Marchetti, S., Pluim, D., Beijnen, J.H. et al. “Effect of the drug transporters ABCB1, ABCC2, and ABCG2 on the disposition and brain accumulation of the taxane analog BMS-275,183”. Invest New Drugs 32, 1083–1095 (2014). https://doi.org/10.1007/s10637-014-0143-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0143-0