Summary
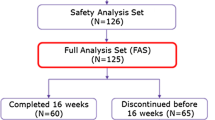
This open-labeled phase II, efficacy-finding study evaluated the efficiency and safety of Pistacia terebinthus soap in metastatic colorectal cancer patients who developed cetuximab induced skin toxicity. Patients who received cetuximab plus chemotherapy and developed Grade 2 or 3 skin toxicity were treated twice daily with a soap made of oil extracted from Pistacia terebinthus. During treatment, no topical or oral antibiotics, corticosteroids or other moisturizers were used. Patients were examined 1 week later and their photographs were taken. Fifteen mCRC patients who developed skin toxicity while receiving first-line CTX in combination with chemotherapy were included into the study. Eight patients were male and the median age was 58 (25–70). Sixty percent of the patients (n:9) had Grade 3 skin toxicity. Complete response rates in patients with Grade 2 and Grade 3 skin toxicities were 100 and 33 %, respectively. In the remaining patients with Grade 3 toxicity the skin toxicity regressed to Grade 1. The objective response rate was 100 %, and no delay, dose reduction or discontinuation of CTX treatment due to skin toxicity was necessary. Skin toxicity reoccurred in all patients when patients stopped administering the soap and therefore they used it throughout the cetuximab treatment. Pistacia terebinthus soap seemed to be used safely and effectively in the treatment of skin toxicity induced by Cetuximab.


Similar content being viewed by others
References
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345
Galimont-Collen AF, Vos LE, Lavrijsen AP, Ouwerkerk J, Gelderblom H (2007) Classification and management of skin, hair, nail and mucosal side-effects of epidermal growth factor receptor (EGFR) inhibitors. Eur J Cancer 43:845–851
Perez-Soler R, Saltz L (2005) Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol: Off J Am Soc Clin Oncol 23:5235–5246
Segaert S, Van Cutsem E (2005) Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol: Off J Eur Soc Med Oncol 16:1425–1433
Di Leo A, Gomez HL, Aziz Z, Zvirbule Z, Bines J, Arbushites MC, Guerrera SF, Koehler M, Oliva C, Stein SH, Williams LS, Dering J, Finn RS, Press MF (2008) Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 26:5544–5552
Lacouture ME, Laabs SM, Koehler M, Sweetman RW, Preston AJ, Di Leo A, Gomez HL, Salazar VM, Byrne JA, Koch KM, Blackwell KL (2009) Analysis of dermatologic events in patients with cancer treated with lapatinib. Breast Cancer Res Treat 114:485–493
Lacouture ME (2006) Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer 6:803–812
Hidalgo M, Siu LL, Nemunaitis J, Rizzo J, Hammond LA, Takimoto C, Eckhardt SG, Tolcher A, Britten CD, Denis L, Ferrante K, Von Hoff DD, Silberman S, Rowinsky EK (2001) Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol Off J Am Soc Clin Oncol 19:3267–3279
Busam KJ, Capodieci P, Motzer R, Kiehn T, Phelan D, Halpern AC (2001) Cutaneous side-effects in cancer patients treated with the antiepidermal growth factor receptor antibody C225. Br J Dermatol 144:1169–1176
Van Doorn R, Kirtschig G, Scheffer E, Stoof TJ, Giaccone G (2002) Follicular and epidermal alterations in patients treated with ZD1839 (Iressa), an inhibitor of the epidermal growth factor receptor. Br J Dermatol 147:598–601
Albanell J, Rojo F, Averbuch S, Feyereislova A, Mascaro JM, Herbst R, LoRusso P, Rischin D, Sauleda S, Gee J, Nicholson RI, Baselga J (2002) Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: histopathologic and molecular consequences of receptor inhibition. J Clin Oncol Off J Am Soc Clin Oncol 20:110–124
Woodworth CD, Michael E, Marker D, Allen S, Smith L, Nees M (2005) Inhibition of the epidermal growth factor receptor increases expression of genes that stimulate inflammation, apoptosis, and cell attachment. Mol Cancer Ther 4:650–658
Robert C, Soria JC, Spatz A, Le Cesne A, Malka D, Pautier P, Wechsler J, Lhomme C, Escudier B, Boige V, Armand JP, Le Chevalier T (2005) Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 6:491–500
Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P (2009) Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 360:1408–1417
Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA, Eastern Cooperative Oncology G (2005) Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol Off J Am Soc Clin Oncol 23:8646–8654
Giro C, Berger B, Bolke E, Ciernik IF, Duprez F, Locati L, Maillard S, Ozsahin M, Pfeffer R, Robertson AG, Langendijk JA, Budach W (2009) High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: results of a survey in EORTC institutes. Radiother Oncol: J Eur Soc Ther Radiol Oncol 90:166–171
Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol Off J Am Soc Clin Oncol 27:663–671
Xiong HQ, Rosenberg A, LoBuglio A, Schmidt W, Wolff RA, Deutsch J, Needle M, Abbruzzese JL (2004) Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II Trial. J Clin Oncol Off J Am Soc Clin Oncol 22:2610–2616
Perez-Soler R (2006) Rash as a surrogate marker for efficacy of epidermal growth factor receptor inhibitors in lung cancer. Clin Lung Cancer 8(Suppl 1):S7–S14
Gatzemeier U, von Pawel J, Vynnychenko I, Zatloukal P, de Marinis F, Eberhardt WE, Paz-Ares L, Schumacher KM, Goddemeier T, O’Byrne KJ, Pirker R (2011) First-cycle rash and survival in patients with advanced non-small-cell lung cancer receiving cetuximab in combination with first-line chemotherapy: a subgroup analysis of data from the FLEX phase 3 study. Lancet Oncol 12:30–37
Petrelli F, Borgonovo K, Barni S (2013) The predictive role of skin rash with cetuximab and panitumumab in colorectal cancer patients: a systematic review and meta-analysis of published trials. Target Oncol 8:173–181
Van Cutsem E, Tejpar S, Vanbeckevoort D, Peeters M, Humblet Y, Gelderblom H, Vermorken JB, Viret F, Glimelius B, Gallerani E, Hendlisz A, Cats A, Moehler M, Sagaert X, Vlassak S, Schlichting M, Ciardiello F (2012) Intrapatient cetuximab dose escalation in metastatic colorectal cancer according to the grade of early skin reactions: the randomized EVEREST study. J Clin Oncol Off J Am Soc Clin Oncol 30:2861–2868
Tuzlaci E, Aymaz PE (2001) Turkish folk medicinal plants, Part IV: Gonen (Balikesir). Fitoterapia 72:323–343
Giner-Larza EM, Manez S, Recio MC, Giner RM, Prieto JM, Cerda-Nicolas M, Rios JL (2001) Oleanonic acid, a 3-oxotriterpene from Pistacia, inhibits leukotriene synthesis and has anti-inflammatory activity. Eur J Pharmacol 428:137–143
Duru ME, Cakir A, Kordali S, Zengin H, Harmandar M, Izumi S, Hirata T (2003) Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia 74:170–176
Wagner LI, Wenzel L, Shaw E, Cella D (2007) Patient-reported outcomes in phase II cancer clinical trials: lessons learned and future directions. J Clin Oncol Off J Am Soc Clin Oncol 25:5058–5062
Lacouture ME, Anadkat MJ, Bensadoun RJ, Bryce J, Chan A, Epstein JB, Eaby-Sandy B, Murphy BA, Group MSTS (2011) Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer: Off J Multinatl Assoc Support Care Cancer 19:1079–1095
Stintzing S, Kapaun C, Laubender RP, Jung A, Neumann J, Modest DP, Giessen C, Moosmann N, Wollenberg A, Kirchner T, Heinemann V (2013) Prognostic value of cetuximab-related skin toxicity in metastatic colorectal cancer patients and its correlation with parameters of the epidermal growth factor receptor signal transduction pathway: results from a randomized trial of the GERMAN AIO CRC Study Group. Int J Cancer J Int Cancer 132:236–245
Davis PH (1967) Flora of Turkey and the East Aegean Islands, vol 2. Edinburgh at the University Press, Edinburgh, pp 546–547
Marone P, Bono L, Leone E, Bona S, Carretto E, Perversi L (2001) Bactericidal activity of Pistacia lentiscus mastic gum against Helicobacter pylori. J Chemother 13:611–614
Kivcak B, Akay S (2005) Quantitative determination of alpha-tocopherol in Pistacia lentiscus, Pistacia lentiscus var. chia, and Pistacia terebinthus by TLC-densitometry and colorimetry. Fitoterapia 76:62–66
Scope A, Agero AL, Dusza SW, Myskowski PL, Lieb JA, Saltz L, Kemeny NE, Halpern AC (2007) Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol: Off J Am Soc Clin Oncol 25:5390–5396
Scope A, Lieb JA, Dusza SW, Phelan DL, Myskowski PL, Saltz L, Halpern AC (2009) A prospective randomized trial of topical pimecrolimus for cetuximab-associated acnelike eruption. J Am Acad Dermatol 61:614–620
Jatoi A, Thrower A, Sloan JA, Flynn PJ, Wentworth-Hartung NL, Dakhil SR, Mattar BI, Nikcevich DA, Novotny P, Sekulic A, Loprinzi CL (2010) Does sunscreen prevent epidermal growth factor receptor (EGFR) inhibitor-induced rash? Results of a placebo-controlled trial from the North Central Cancer Treatment Group (N05C4). Oncologist 15:1016–1022
Jatoi A, Rowland K, Sloan JA, Gross HM, Fishkin PA, Kahanic SP, Novotny PJ, Schaefer PL, Johnson DB, Tschetter LK, Loprinzi CL (2008) Tetracycline to prevent epidermal growth factor receptor inhibitor-induced skin rashes: results of a placebo-controlled trial from the North Central Cancer Treatment Group (N03CB). Cancer 113:847–853
Lacouture ME, Mitchell EP, Piperdi B, Pillai MV, Shearer H, Iannotti N, Xu F, Yassine M (2010) Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol Off J Am Soc Clin Oncol 28:1351–1357
Wehler TC, Graf C, Mohler M, Herzog J, Berger MR, Gockel I, Lang H, Theobald M, Galle PR, Schimanski CC (2013) Cetuximab-induced skin exanthema: prophylactic and reactive skin therapy are equally effective. J Cancer Res Clin Oncol 139:1667–1672
Wong SF, Lindgren A, Mummaneni M, Byun T, Vasko C, Arenos R, Alexson E, Osann K (2010) A prospective crossover pilot study to evaluate the use of a topical wound gel in patients with cutaneous toxicity caused by epidermal growth factor receptor inhibitors. J Support Oncol 8:202–208
Katzer K, Tietze J, Klein E, Heinemann V, Ruzicka T, Wollenberg A (2010) Topical therapy with nadifloxacin cream and prednicarbate cream improves acneiform eruptions caused by the EGFR-inhibitor cetuximab - a report of 29 patients. Eur J Dermatol 20:82–84
Ocvirk J (2010) Management of cetuximab-induced skin toxicity with the prophylactic use of topical vitamin K1 cream. Radiol Oncol 44:265–266
Acknowledgments
This work has no funding source.
Ethical standards
The study was performed in accordance with the Declaration of Helsinki (5th revision, October 2000) of the World Medical Association and approved by the National Medical Ethics Committee of the Republic of Turkey. All patients signed informed consent forms.
Conflicts of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tastekin, D., Tambas, M., Kilic, K. et al. The efficacy of Pistacia Terebinthus soap in the treatment of cetuximab-induced skin toxicity. Invest New Drugs 32, 1295–1300 (2014). https://doi.org/10.1007/s10637-014-0128-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0128-z