Summary
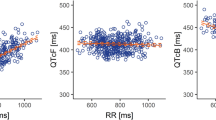
Background Several cancer therapies can prolong cardiac repolarization. This study assessed the potential of eribulin to affect cardiac repolarization in patients with advanced solid tumors. Methods In this Phase I, open-label, single-arm study, patients received eribulin mesylate (1.4 mg/m2; Days 1 and 8 of a 21-day cycle). The primary objective was to assess the effect of eribulin on the QTcF pre- and post-infusion; QTcF and QTcNi were compared for ability to remove heart-rate dependence of the QT interval. Relationship between concentration of eribulin and ΔQTc was explored using linear mixed-effects analysis. Secondary objectives explored pharmacokinetics, safety, and tolerability. Results Twenty-six patients were enrolled. QTcNi was more effective than QTcF in correcting for heart-rate dependency of the QT interval. On Day 1, mean ΔQTcNi were ~0 at all timepoints. An apparent time-dependent increase in ΔQTc was observed: on Day 8, changes from baseline were larger and more variable, without clear relation to plasma levels of eribulin. Day 8 predose ΔQTcNi was 5 ms, post-infusion mean values ranged from 2 to 9 ms (largest mean ΔQTcNi at 6 h). No new or unexpected toxicities were reported. Conclusion Eribulin demonstrated an acceptable safety profile and a minor prolongation of QTc not expected to be of clinical concern in oncology patients.





Similar content being viewed by others
References
Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, Littlefield BA, Wilson L (2005) The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther 4:1086–1095
Okouneva T, Azarenko O, Wilson L, Littlefield BA, Jordan MA (2008) Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol Cancer Ther 7:2003–2011
Smith JA, Wilson L, Azarenko O, Zhu X, Lewis BM, Littlefield BA, Jordan MA (2010) Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry 49:1331–1337
Goel S, Mita AC, Mita M, Rowinsky EK, Chu QS, Wong N, Desjardins C, Fang F, Jansen M, Shuster DE, Mani S, Takimoto C (2009) A phase I study of eribulin mesylate (E7389), a mechanistically novel inhibitor of microtubule dynamics, in patients with advanced solid malignancies. Clin Cancer Res 15:4207–4212
Cortes J, Vahdat L, Blum JL, Twelves C, Campone M, Roche H, Bachelot T, Awada A, Paridaens R, Goncalves A, Shuster DE, Wanders J, Fang F, Gurnani R, Richmond E, Cole PE, Ashworth S, Allison MA (2010) Phase II study of the halichondrin B analog eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline, a taxane, and capecitabine. J Clin Oncol 28:3922–3928
Tan AR, Rubin EH, Walton DC, Shuster DE, Wong NY, Fang F, Ashworth S, Rosen LS (2009) Phase I study of eribulin mesylate (E7389) administered once every 21 days in patients with advanced solid tumors. Clin Cancer Res 15:4213–4218
Vahdat LT, Pruitt B, Fabian CJ, Rivera RR, Smith DA, Tan-Chiu E, Wright J, Tan AR, Dacosta NA, Chuang E, Smith J, O’Shaughnessy J, Shuster DE, Meneses NL, Chandrawansa K, Fang F, Cole PE, Ashworth S, Blum JL (2009) Phase II study of eribulin mesylate, a halichondrin B analog, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 27:2954–2961
Cortes J, O’Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, Chollet P, Manikas A, Dieras V, Delozier T, Vladimirov V, Cardoso F, Koh H, Bougnoux P, Dutcus CE, Seegobin S, Mir D, Meneses N, Wanders J, Twelves C (2011) Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377:914–923
Murphy CG, Seidman AD (2009) Evolving approaches to metastatic breast cancer previously treated with anthracyclines and taxanes. Clin Breast Cancer 9(Suppl 2):S58–S65
Pean E, Klaar S, Berglund EG, Salmonson T, Borregaard J, Hofland KF, Ersboll J, Abadie E, Giuliani R, Pignatti F (2012) The European medicines agency review of eribulin for the treatment of patients with locally advanced or metastatic breast cancer: summary of the scientific assessment of the committee for medicinal products for human use. Clin Cancer Res 18:4491–4497
Eisai Inc (2011) Halaven prescribing information. http://www.halaven.com/. Accessed 30 March 2011
Strevel EL, Ing DJ, Siu LL (2007) Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol 25:3362–3371
Barbey JT, Pezzullo JC, Soignet SL (2003) Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol 21:3609–3615
Varterasian M, Meyer M, Fingert H, Radlowski D, Asbury P, Zhou X, Healey D (2003) Baseline heart rate-corrected QT and eligibility for clinical trials in oncology. J Clin Oncol 21:3378–3379
Shah RR (2002) Drug-induced prolongation of the QT interval: regulatory dilemmas and implications for approval and labelling of a new chemical entity. Fundam Clin Pharmacol 16:147–156
Shah RR (2002) Drug-induced prolongation of the QT interval: why the regulatory concern? Fundam Clin Pharmacol 16:119–124
Tornoe CW, Garnett CE, Wang Y, Florian J, Li M, Gobburu JV (2011) Creation of a knowledge management system for QT analyses. J Clin Pharmacol 51:1035–1042
International Conference on Harmonisation (2012) International conference on harmonisation E14 guideline: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. http://www.ich.org. Accessed 23 January 2012
Jonsson MK, Vos MA, Duker G, Demolombe S, van Veen TA (2010) Gender disparity in cardiac electrophysiology: implications for cardiac safety pharmacology. Pharmacol Ther 127:9–18
Rock EP, Finkle J, Fingert HJ, Booth BP, Garnett CE, Grant S, Justice RL, Kovacs RJ, Kowey PR, Rodriguez I, Sanhai WR, Strnadova C, Targum SL, Tsong Y, Uhl K, Stockbridge N (2009) Assessing proarrhythmic potential of drugs when optimal studies are infeasible. Am Heart J 157:827–836
Mukohara T, Nagai S, Mukai H, Namiki M, Minami H (2011) Eribulin mesylate in patients with refractory cancers: a phase I study. Invest New Drugs 30:1926–1933
Darpo B (2010) The thorough QT/QTc study 4 years after the implementation of the ICH E14 guidance. Br J Pharmacol 159:49–57
Fossa AA, Wisialowski T, Magnano A, Wolfgang E, Winslow R, Gorczyca W, Crimin K, Raunig DL (2005) Dynamic beat-to-beat modeling of the QT-RR interval relationship: analysis of QT prolongation during alterations of autonomic state versus human ether a-go-go-related gene inhibition. J Pharmacol Exp Ther 312:1–11
Fossa AA, Wisialowski T, Crimin K (2006) QT prolongation modifies dynamic restitution and hysteresis of the beat-to-beat QT-TQ interval relationship during normal sinus rhythm under varying states of repolarization. J Pharmacol Exp Ther 316:498–506
Malik M (2001) Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol 12:411–420
Devriese LA, Mergui-Roelvink M, Wanders J, Jenner A, Edwards G, Reyderman L, Copalu W, Peng F, Marchetti S, Beijnen JH, Schellens JH (2012) Eribulin mesylate pharmacokinetics in patients with solid tumors receiving repeated oral ketoconazole. Invest New Drugs. doi:10.1007/s10637-012-9829-3
Bagnes C, Panchuk PN, Recondo G (2010) Antineoplastic chemotherapy induced QTc prolongation. Curr Drug Saf 5:93–96
Kitagawa K, Kawada K, Morita S, Inada M, Mitsuma A, Sawaki M, Iino S, Inden Y, Murohara T, Imai T, Ando Y (2012) Prospective evaluation of corrected QT intervals and arrhythmias after exposure to epirubicin, cyclophosphamide, and 5-fluorouracil in women with breast cancer. Ann Oncol 23:743–747
Acknowledgments
We would like to thank all of the patients, and the investigators and their teams, who participated in these studies. We also thank Biotrial (Rennes, France), which provided logistical support for this study, and Varinia Munoz, PhD, from Complete Medical Communications, who provided medical writing support funded by Eisai Inc.
Disclosures
L. Reyderman and A. Kristensen are employees of Eisai Inc. and K. Law is an employee of Eisai Ltd. At the time of study, J. Wanders and G. Edwards were employees of Eisai Ltd. T. Lesimple has received research funding from Roche and Bristol-Myers Squibb, and has acted as a consultant for Roche. B. Darpo has acted as a consultant for Mangan AB. J. Edeline, T.J. Carrothers, F. Cvitkovic, J. P. Delord, H. Léna and N. Penel have no potential conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial support
This study was funded by Eisai Inc.
Rights and permissions
About this article
Cite this article
Lesimple, T., Edeline, J., Carrothers, T.J. et al. A phase I, open-label, single-arm study for QT assessment of eribulin mesylate in patients with advanced solid tumors. Invest New Drugs 31, 900–909 (2013). https://doi.org/10.1007/s10637-012-9893-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-012-9893-8