Summary
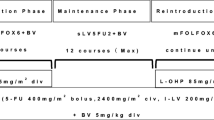
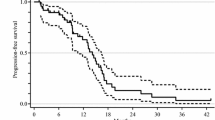
Background To investigate the efficacy and safety of bevacizumab beyond first progression combined with doublet chemotherapy in patients with metastatic colorectal cancer. Methods This multicenter phase II study included 76 patients with metastatic colorectal cancer progressed after first-line bevacizumab plus doublet chemotherapy. Study treatment consisted of second-line continuation of bevacizumab plus crossover standard doublet chemotherapy, consisting of FOLFOX, CapeOX, or FOLFIRI. Bevacizumab was administered in doses of 5 mg/kg/2-week or 7.5 mg/kg/3-week according to the schedules of the combined regimen. Results Median progression-free survival (PFS) and overall survival (OS) was 6.5 months (95 % CI, 5.2–7.8) and 12.8 months (95 % CI, 8.8–16.9), respectively, with no significant differences according to combined doublet chemotherapy. The response rate (RR) was 17.1 % (95 % CI, 8.6–5.6) with no statistical significance between regimens (p = 0.053). The first-line RR and PFS did not affect the second-line efficacy outcomes; RR (14.0 % vs 21.2 %, p = 0.405), median PFS (5.6 vs 6.7 months, p = 0.335), and OS (15.4 vs 11.0 months, p = 0.383) were not different between previous responders and non-responders, and the median PFS (p = 0.186) and OS (p = 0.495) were not different either according to the length of first-line PFS; however, OS from the first-line chemotherapy was longer in patients with longer first-line PFS (26.4 vs 14.8 months, p = 0.010). Bevacizumab-related significant adverse events included proteinuria (1.3 %) and thromboembolism (1.3 %). Conclusions Bevacizumab beyond first progression could be considered a treatment strategy even in patients progressed after first-line bevacizumab plus doublet chemotherapy. Second-line efficacy outcomes did not differ according to the first-line responses.



Similar content being viewed by others
References
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, Hendler D, de Braud F, Wilson C, Morvan F, Bonetti A (2000) Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 18(16):2938–2947
Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355(9209):1041–1047
Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB 3rd (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25(12):1539–1544. doi:10.1200/JCO.2006.09.6305
Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, Kozloff M (2008) Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol 26(33):5326–5334. doi:10.1200/JCO.2008.16.3212
Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr Y, Saif MW, Schwartzberg L, Hedrick E (2008) Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol 26(21):3523–3529. doi:10.1200/JCO.2007.15.4138
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. doi:10.1056/NEJMoa032691
Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26(12):2013–2019. doi:10.1200/JCO.2007.14.9930
Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, Landi B, Colin P, Louvet C, de Gramont A (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22(2):229–237. doi:10.1200/JCO.2004.05.113
Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29(15):2011–2019. doi:10.1200/JCO.2010.33.5091
Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, Dibartolomeo M, Mazier MA, Canon JL, Georgoulias V, Peeters M, Bridgewater J, Cunningham D (2009) Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. doi:10.1093/annonc/mdp233
Bekaii-Saab TS, Grothey A, Bendell JC, Kozloff M, Cohn AL, Mun Y, Fish S, Flick ED, Dalal D, Hurwitz H (2012) Effectiveness and safety of second-line (2L) irinotecan- and oxaliplatin-based regimens after first-line (1L) bevacizumab (BV)-containing treatment (tx) for metastatic colorectal cancer (mCRC): results from the ARIES observational cohort study. ASCO Meet Abstr 30(4_suppl):535
Kozloff M, Bekaii-Saab TS, Bendell JC, Cohn AL, Hurwitz H, Roach N, Tezcan H, Fish S, Flick ED, Mun Y, Dalal D, Grothey A (2011) Effectiveness of first- or second-line bevacizumab (BV) treatment (tx) in elderly patients (pts) with metastatic colorectal cancer (mCRC) in ARIES, an observational cohort study (OCS). ASCO Meet Abstr 29(15_suppl):3625
Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS (2000) Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Investig 105(8):R15–R24. doi:10.1172/JCI8829
Klement G, Huang P, Mayer B, Green SK, Man S, Bohlen P, Hicklin D, Kerbel RS (2002) Differences in therapeutic indexes of combination metronomic chemotherapy and an anti-VEGFR-2 antibody in multidrug-resistant human breast cancer xenografts. Clin Cancer Res: Off J Am Assoc Cancer Res 8(1):221–232
Kopetz S, Abbruzzese JL (2009) Hidden biases in an observational study of bevacizumab beyond progression. J Clin Oncol 27(10):1732–1733. doi:10.1200/JCO.2009.21.2084, author reply 1733
Rothenberg ML, Cox JV, Butts C, Navarro M, Bang YJ, Goel R, Gollins S, Siu LL, Laguerre S, Cunningham D (2008) Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Ann Oncol 19(10):1720–1726. doi:10.1093/annonc/mdn370
Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, Andre T, Chan E, Lordick F, Punt CJ, Strickland AH, Wilson G, Ciuleanu TE, Roman L, Van Cutsem E, Tzekova V, Collins S, Oliner KS, Rong A, Gansert J (2010) Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 28(31):4706–4713. doi:10.1200/JCO.2009.27.6055
Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, Lenz HJ, Borg C, Middleton G, Kroning H, Luppi G, Kisker O, Zubel A, Langer C, Kopit J, Burris HA 3rd (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26(14):2311–2319. doi:10.1200/JCO.2007.13.1193
Tabernero J, Van Cutsem E, Lakomy R, Prausova J, Ruff P, Van Hazel G, Moiseyenko V, Ferry D, McKendrick J, Soussan-Lazard K, Boelle E, Allegra C (2011) Results from VELOUR, a phase 3 study of aflibercept (A) versus placebo (pbo) in combination with FOLFIRI for the treatment of patients (pt) with previously treated metastatic colorectal cancer (MCRC). Eur J Cancer 47(Supplement 2 (0)):5. doi:10.1016/s0959-8049(11)70105-8
Horita Y, Yamada Y, Kato K, Hirashima Y, Akiyoshi K, Nagashima K, Nakajima T, Hamaguchi T, Shimada Y (2011) Phase II clinical trial of second-line FOLFIRI plus bevacizumab for patients with metastatic colorectal cancer: AVASIRI trial. Int J Clin Oncol / Japan Soc Clin Oncol. doi:10.1007/s10147-011-0331-2
Arnold D, Andre T, Bennouna J, Sastre J, Osterlund PJ, Greil R, Van Cutsem E, Von Moos R, Reyes-Rivera I, Bendahmane B, Kubicka S (2012) Bevacizumab (BEV) plus chemotherapy (CT) continued beyond first progression in patients with metastatic colorectal cancer (mCRC) previously treated with BEV plus CT: results of a randomized phase III intergroup study (TML study). ASCO Meet Abstr 30(18_suppl):CRA3503
Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, Langer C, Moore MJ, Zalcberg JR (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359(17):1757–1765. doi:10.1056/NEJMoa0804385
Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25(13):1658–1664. doi:10.1200/JCO.2006.08.1620
Mishima H, Oba K, Sakamoto J, Muro K, Yoshino T, Hyodo I, Maehara Y (2012) FOLFIRI plus bevacizumab 5 mg/kg versus 10 mg/kg as second-line therapy in patients with metastatic colorectal cancer who have failed first-line bevacizumab plus oxaliplatin-based therapy: a randomized phase III study (EAGLE Study). Jpn J Clin Oncol 42(2):134–138. doi:10.1093/jjco/hyr180
Acknowledgments
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065 and A062254). Bevacizumab was kindly provided by Roche Korea Co., Ltd.
Conflict of interest statement
We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yong Sang Hong and Jeeyun Lee equally contributed to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table
Treatment exposure. (DOC 45 kb)
Rights and permissions
About this article
Cite this article
Hong, Y.S., Lee, J., Kim, Kp. et al. Multicenter phase II study of second-line bevacizumab plus doublet combination chemotherapy in patients with metastatic colorectal cancer progressed after upfront bevacizumab plus doublet combination chemotherapy. Invest New Drugs 31, 183–191 (2013). https://doi.org/10.1007/s10637-012-9853-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-012-9853-3