Summary
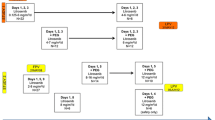
Purpose This phase I study aims at assessing the safety and tolerability of LY2603618, a selective inhibitor of Checkpoint Kinase 1, in combination with pemetrexed and determining the maximum tolerable dose and the pharmacokinetic parameters. Experimental design This was an open-label, multicenter, dose-escalation study in patients with advanced solid tumors. Increasing doses of LY2603618 (40–195 mg/m2) were combined with 500 mg/m2 of pemetrexed. LY2603618 was administered on Days 1 and 9 and pemetrexed on Day 8 in a 28-day cycle. For all subsequent 21-day cycles, pemetrexed was administered on Day 1 and LY2603618 on Day 2. Antitumor activity was evaluated as per Response Evaluation Criteria in Solid Tumors 1.0. Results A total of 31 patients were enrolled into six cohorts (three at 40 mg/m2 over 4.5-hour infusion, 1-hour infusion in subsequent cohorts: three each at 40 mg/m2, 70 mg/m2, and 195 mg/m2; 13 at 105 mg/m2; six at 150 mg/m2). Four patients experienced a dose-limiting toxicity: diarrhea (105 mg/m2); reversible infusion-related reaction (150 mg/m2); thrombocytopenia (195 mg/m2); and fatigue (195 mg/m2). The maximum tolerated dose was defined as 150 mg/m2. The pharmacokinetic data demonstrated that the exposure of LY2603618 increased in a dose-dependent manner, displayed a suitable half-life for maintaining required human exposures while minimizing the intra- and inter-cycle accumulation, and was unaffected by the pemetrexed administration. The pharmacokinetic-defined biologically efficacious dose was achieved at doses ≥105 mg/m2. Conclusion LY2603618 administered approximately 24 h after pemetrexed showed acceptable safety and pharmacokinetic profiles.




Similar content being viewed by others
References
Miller KD, Picus J, Blanke C et al (2000) Phase II study of the multitargeted antifolate LY231514 (ALIMTA, MTA, pemetrexed disodium) in patients with advanced pancreatic cancer. Ann Oncol 11:101–103
Sanchez Y, Wong C, Thoma RS et al (1997) Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277:1497–1501
Karnitz LM, Flatten KS, Wagner JM et al (2005) Gemcitabine-induced activation of checkpoint signaling pathways that affect tumor cell survival. Mol Pharmacol 68:1636–1644
Liu Q, Guntuku S, Cui XS et al (2000) Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev 14:1448–1459
Morgan MA, Parsels LA, Parsels JD, Mesiwala AK, Maybaum J, Lawrence TS (2005) Role of checkpoint kinase 1 in preventing premature mitosis in response to gemcitabine. Cancer Res 65:6835–6842
Zhao H, Watkins JL, Piwnica-Worms H (2002) Disruption of the checkpoint kinase 1/cell division cycle 25A pathway abrogates ionizing radiation-induced S and G2 checkpoints. Proc Natl Acad Sci USA 99:14795–14800
Dai Y, Grant S (2010) New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin Cancer Res 16:376–383
Tonkinson JL, Marder P, Andis SL et al (1997) Cell cycle effects of antifolate antimetabolites: implications for cytotoxicity and cytostasis. Cancer Chemother Pharmacol 39:521–531
Tonkinson JL, Worzalla JF, Teng CH, Mendelsohn LG (1999) Cell cycle modulation by a multitargeted antifolate, LY231514, increases the cytotoxicity and antitumor activity of gemcitabine in HT29 colon carcinoma. Cancer Res 59:3671–3676
Merry C, Fu J, Wang J, Yeh I-Ju, Zhang Y (2010) Targeting the checkpoint kinase Chk1 in cancer therapy. Cell Cycle 9:279–283
Tse AN, Carvajal R, Schwartz GK (2007) Targeting checkpoint kinase 1 in cancer therapeutics. Clin Cancer Res 13:1955–1960
Marshall M, Barda D, Barnard D et al (2009) Characterization and preclinical development of LCI-1, a selective and potent Chk1 inhibitor in phase I clinical trials. Mol Cancer Ther 8(12 suppl 1):B248
Van Triest B, Pinedo HM, Giaccone G, Peters GJ (2000) Downstream molecular determinants of response to 5-fluorouracil and antifolate thymidylate synthase inhibitors. Ann Oncol 11:385–391
Giovannetti E, Honeywell R, Hanauske AR et al (2010) Pharmacological aspects of the enzastaurin-pemetrexed combination in non-small cell lung cancer (NSCLC). Curr Drug Targets 11:12–28
Marshall M, Barnard D, Diaz B et al. (2010) Evaluation of the antitumor activity of pemetrexed in combination with the Chk1 inhibitor LY2603618. Poster presented at 22nd EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, Berlin, Germany, Abstract No. 201
Hanauske AR, Lahn M, Musib LC et al (2009) Phase Ib safety and pharmacokinetic evaluation of daily and twice daily oral enzastaurin in combination with pemetrexed in advanced/metastatic cancer. Ann Oncol 20:1565–1575
Rollins KD, Lindley C (2005) Pemetrexed: a multitargeted antifolate. Clin Ther 27:1343–1382
Boles NC, Peddibhotla S, Chen AJ, Goodell MA, Rosen JM (2010) Chk1 haploinsufficiency results in anemia and defective erythropoiesis. PLoS One 5:e8581
Kummar S, Gutierrez ME, Gardner ER et al (2010) A phase I trial of UCN-01 and prednisone in patients with refractory solid tumors and lymphoma. Cancer Chemother Pharmacol 65:383–389
Cullen MH, Zatloukal P, Sörenson S et al (2008) A randomized phase III trial comparing standard and high-dose pemetrexed as second-line treatment in patients with locally advanced or metastatic non-small-cell lung cancer. Ann Oncol 19:939–945
Cohen MH, Johnson JR, Wang YC, Sridhara R, Pazdur R (2005) FDA drug approval summary: pemetrexed for injection (Alimta) for the treatment of non-small cell lung cancer. Oncologist 10:363–368
Acknowledgements
This phase I study was funded by Eli Lilly and Company (Study Code: I2I-MC-JMMB). We thank the patients who participated in this trial and the study coordinators, nurse practitioners, clinical research assistants, and doctors who provided valuable assistance in this study. We also thank Dr. Richard Gaynor for his interest and support, Rodney Decker for pharmacokinetic analyst support, and Kate Trenor for project management support and services with Novella Clinical (formerly Prologue Research International), Columbus, OH. We would like to acknowledge the medical writing assistance provided by PRIMO Scientific Corporation, Panama, Rep. of Panama.
Conflicts of interest
GJ Weiss is a consultant/advisory board member of Merrimack Pharmaceuticals, Cephalon, Inc., Eli Lilly and Genzyme Corporation.
Author information
Authors and Affiliations
Corresponding author
Additional information
The results of this study have been reported at the 22nd EORTC-NCI-AACR symposium on “Molecular Targets and Cancer Therapeutics”; Berlin, Germany; November 16–19, 2010.
Rights and permissions
About this article
Cite this article
Weiss, G.J., Donehower, R.C., Iyengar, T. et al. Phase I dose-escalation study to examine the safety and tolerability of LY2603618, a checkpoint 1 kinase inhibitor, administered 1 day after pemetrexed 500 mg/m2 every 21 days in patients with cancer. Invest New Drugs 31, 136–144 (2013). https://doi.org/10.1007/s10637-012-9815-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-012-9815-9